More results...
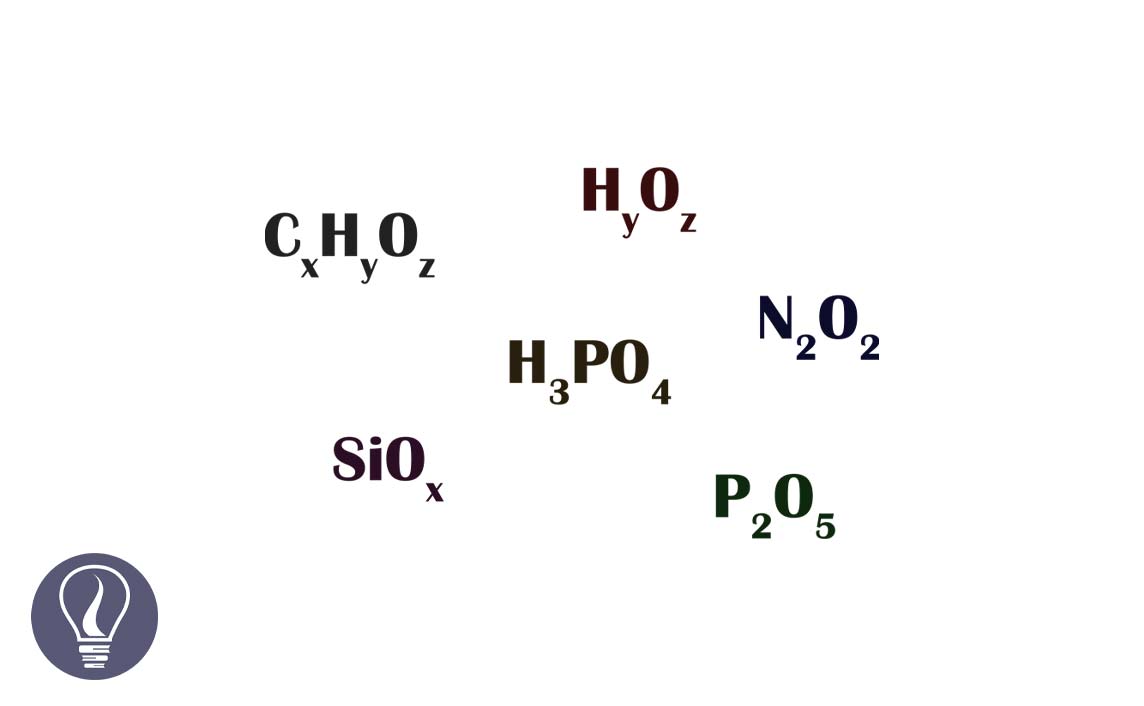

The simplest ratio between the number of mols of atoms in one mole of a compound is known as the empirical formula. In other words, the empirical formula is the simplest ratio between the number of atoms in one molecule. The empirical formula gives an idea about the percentage of each element in a compound.
The formula that shows the exact number of moles of atoms contained in one mole of a compound is called the molecular formula. Or molecular formula shows the exact number of each atom in a molecule.
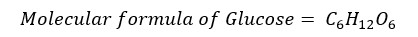
In a glucose molecule, the number ratio between C: H: O is 1:2:1. Therefore, the empirical formula of glucose is as follows.
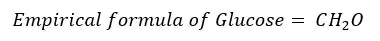
The molecular formula shows the exact number of atoms in a molecule whereas the empirical formula shows the simplest ratio between the atoms. But for some compounds, both the empirical formula and molecular formula are the same. The relation between the empirical formula and molecular formula can be expressed as follows.
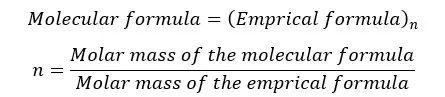
"n" is always a whole number. (n = 1,2,3,…)
There can be several molecular formulas for one empirical formula. As an example, CH2 empirical formulas have different molecular formulas like C2H4, C3H6, C4H8, etc.
If there are several molecular formulas for one empirical formula, the mass percentages of elements in each formula are the same. Therefore, the Carbon percentage and Hydrogen percentage of above C2H4, C3H6, and C4H8 are the same. Therefore, when we know the mass percentage of a particular compound, both the empirical formula and molecular formula can be derived.
Simply, the empirical and molecular formulas give the mole ratio between atoms in a compound. When the mass percentages of each element in a compound are known, they should be converted into mole ratios to derive the empirical formula.
The question:
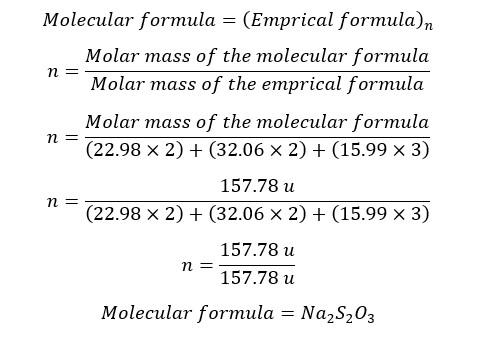
An inorganic compound contains only Sodium (Na), Sulfur (S), and Oxygen (O). Mass percentages of those elements are, Na – 29.11%, S - 40.50%, and O - 30.39%. the relative molar mass of the compound is 157.78 u. Relative atomic masses of Na, S, and O are 22.98 u, 32.06 u, and 15.99 u respectively. Find the empirical and molecular formula of the above compound.
The answer:
| Element | Na | S | O |
| Mass ratio | 29.11 | 40.50 | 30.39 |
| Element | Na | S | O |
| Mole ratio | 29.11/22.98 | 40.50/32.06 | 30.39/15.99 |
| 1.26 | 1.26 | 1.90 |
| Element | Na | S | O |
| Mole ratio | 1.26/1.26 | 1.26/1.26 | 1.90/1.26 |
| 1 | 1 | 1.5 |
| Element | Na | S | O |
| Mole ratio | 1×2 | 1×2 | 1.5×2 |
| 2 | 2 | 3 |
The question:
x.H2O is a white color crystalline salt. X contains 19.4% of carbon, 6.4% of hydrogen, 22.6% of nitrogen, and 51.6% of oxygen. Derive the empirical formula of x. (Relative atomic masses; C - 12.01 u, H - 1.00 u, N - 14.00 u, O - 15.99 u)
If the above compound releases 2 mols of Ammonia gas (NH4) out of 1mol of the compound when it is heated find the molecular formula of the compound. Consider NH3 is the only product released from compound x, that contains nitrogen (N).
The answer:
| Element | C | H | N | O |
| Mass ratio | 19.4 | 6.4 | 22.6 | 51.6 |
| Mole ratio | 19.4/12.01 | 6.4/1.00 | 22.6/14.00 | 51.6/15.99 |
| 1.61 | 6.4 | 1.61 | 3.22 | |
| Divide by the smallest number | 1.61/1.61 | 6.4/1.61 | 1.61/1.61 | 3.22/1.61 |
| 1 | 3.97 | 1 | 2 | |
| Round to the nearest whole number | 1 | 4 | 1 | 2 |
1 mole of compound releases two mols of NH4 means that there are two mols of Nitrogen molecules in one mol of the compound. The empirical formula contains only one mole of nitrogen. Therefore, the empirical formula should be multiplied by 2 to get the molecular formula.
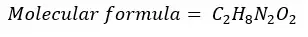

Chem.libretexts.org - Calculating Empirical Formulas for Compounds