More results...

Early in the 20th century, chemical bonding was described according to the Valence bond theory (VBT). VBT explains that atomic orbitals like s, p, and d have undergone hybridization and form hybrid orbitals like sp, sp2, sp3, sp2d1, etc. However, this theory could not explain the energy of electrons, the tetravalency, or Carbon and this theory assumes that electrons are localized.
With the failure of VBT, The Molecular Orbital Theory has been developed. In MOT chemical bonding of a molecule is described using hypothetical molecular orbitals which are bonding molecular orbitals and antibonding molecular orbitals. Here the hybridization of orbitals is not described.
In MOT, molecular orbitals are described that they are formed through a linear combination of atomic orbitals (LCAO). LCAO is used to describe the formation of molecular orbitals when atoms bond to form molecules. For a proper combination, atomic orbitals should have the same energy and same symmetry.
When two atomic orbitals come together to form molecular orbitals, the number of orbitals is always conserved. That means the number of atomic orbitals is equal to the number of molecular orbitals.
There will be formed bonding molecular orbitals, antibonding molecular orbitals, and non-bonding orbitals. Bonding molecular orbitals always have low energy than atomic orbitals whereas antibonding molecular orbitals have higher energy than atomic orbitals.
Molecular orbitals are filled with electrons from the orbitals with the lowest energy to the orbitals with the higher energy.
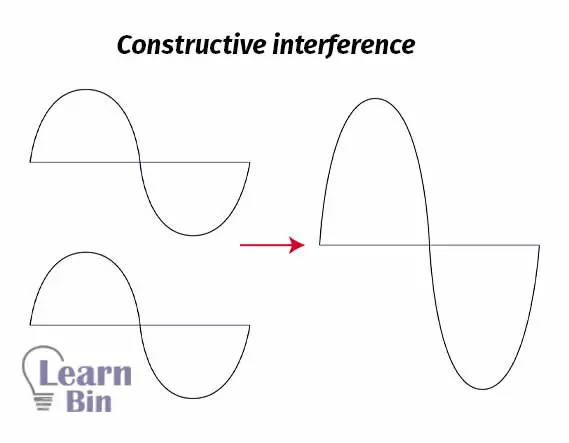
Electrons can be described as waves. If two waves that are in phase add together, it will create constructive interference. That means a larger wave. If two atoms contain electrons that are in phase, the same phenomenon happens. Two electrons in the same phase are combined and form molecular orbitals.
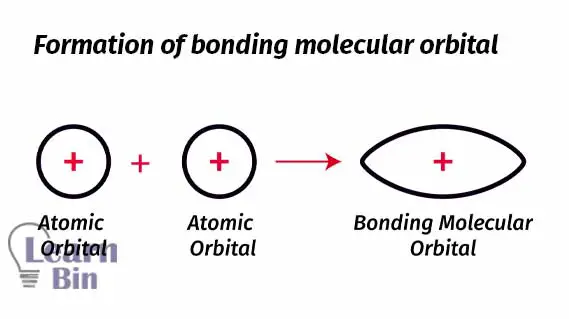
The area of molecular orbital represents a high probability of finding an electron. The molecular orbital is formed between the two nuclei. So, there is a high probability of finding an electron between two nuclei. If there are two electrons between two nuclei, it tends to form a molecule.
To get a proper understanding, let’s take the Hydrogen molecule (H2) as an example. In an H2 molecule, there are two nuclei and two electrons. Each nucleus has one proton. The electrons are between the two nuclei.
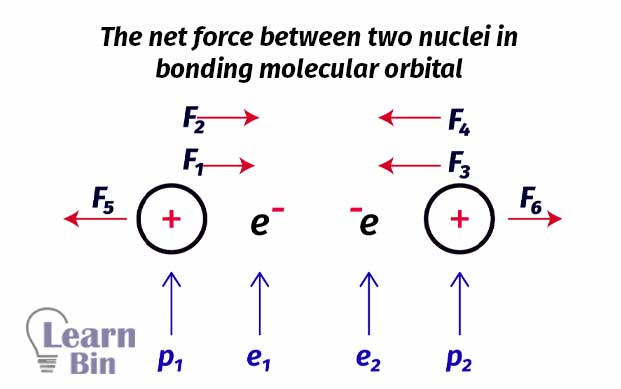
Four forces are pulling protons together and only two forces are pulling forces apart. There are more forces that cause protons to move together rather than pull apart. So, in this type of arrangement, the net force is an attractive force. Therefore, it is always favorable to form a molecule. In other words, filling electrons to the bonding molecular orbitals is favorable for the formation of a molecule.
When two waves, that are out of phase, are combined, and when this happens then destructive interference will occur.
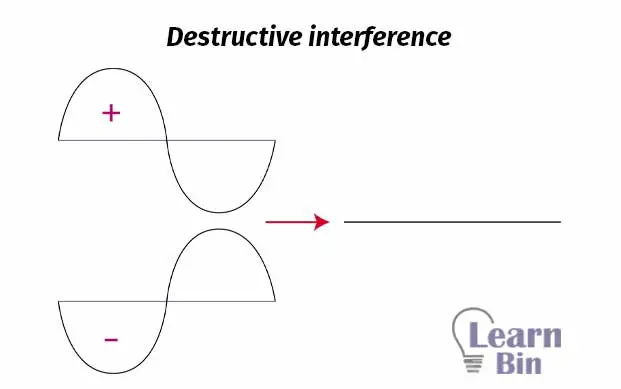
We can take electrons as waves. If we add two electrons out of phase, destructive interference will occur. So, an anti-bonding molecular orbital is formed. Antibonding molecular orbitals are placed beside two atoms. The region between two atoms is the region where destructive interference occurs. This region is known as “Node” and it is the region where the probability of finding an electron is basically zero (probability is very low).
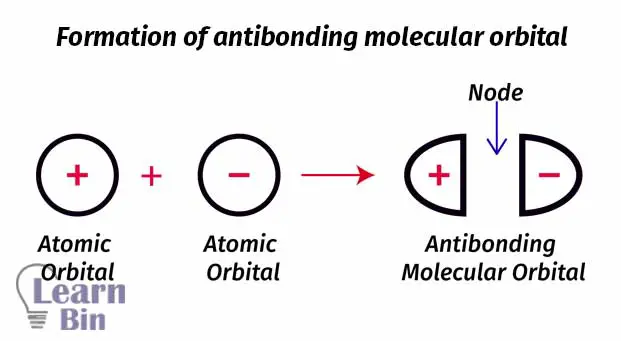
Let’s take two hydrogen atoms to explain the antibonding molecular orbital. The node is placed between the two nuclei of hydrogen atoms. So, there is a very low probability to find an electron between two atoms. Electrons are more likely placed beside the nuclei.
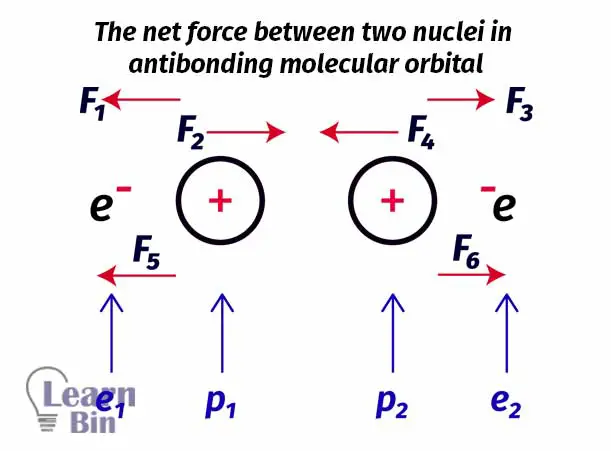
Here only two small forces are pulling the nucleus together and four large forces are pulling the nucleus apart. There are more forces that cause protons to move apart rather than pull together. So, in this type of arrangement, the net force is a repulsive force. Therefore, it is not favorable to form a molecule. In other words, filling electrons to the antibonding molecular orbitals is not favorable for the formation of a molecule.
Nonbonding molecular orbitals have similar energies to their atomic orbitals. The occupation of electrons in nonbonding molecular orbital does not increase or decrease the energy of the molecule. Also, it does not affect the bond order.
In diatomic molecules like HF, 1s atomic orbital in Hydrogen and 2pz atomic orbital in Fluorine are contributed to form molecular orbitals (σ and σ* molecular orbitals). Here, 2px and 2py orbitals have no orbitals to combine with. Therefore, they remain as nonbonding molecular orbitals.
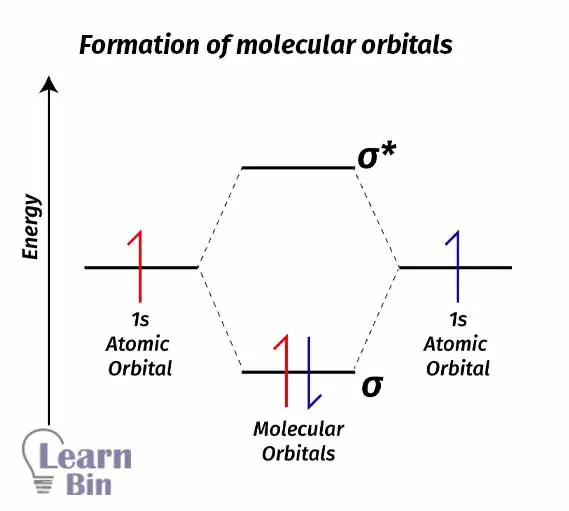
As an example, let’s take the H2 molecule. 1s orbitals in two different Hydrogen atoms will combine and form an H2 molecule. Combining two atomic orbitals will create two molecular orbitals. The molecular orbital with lower energy (than the atomic orbital) is the bonding molecular orbital σ1s and the molecular orbital with higher energy (than the atomic orbital) is the antibonding molecular orbital σ1s*.
Each Hydrogen atom contains one electron with it. As a natural system, electrons always tend to seek to lower energy levels from higher energy levels. So, electrons will fall into a bonding molecular orbital and form an H2 sigma bond. If electrons fall from a higher energy state to a lower energy state, energy is released.
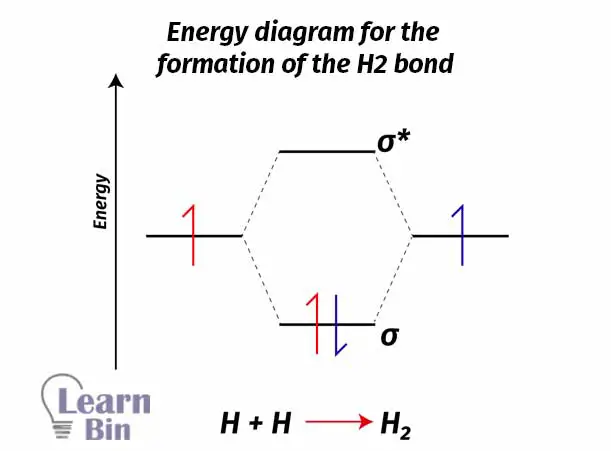
In this situation, two Hydrogen atoms are combined and form an H2 molecule. Energy is released. Therefore, this is an exothermic process.
Magnetic properties of substances are described using diamagnetic and paramagnetic properties. In an induced magnetic field, paramagnetic substances are attracted by the magnetic field whereas diamagnetic substances are repelled by the magnetic field. Paramagnetic substances have unpaired electrons and diamagnetic substances have only paired electrons.
Bond order is the number of chemical bonds between two atoms in a molecule. It is given by the difference between the number of bonding electrons and the number of antibonding electrons divided by 2.
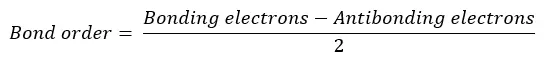
Bond order is an indicator of the stability of a molecule. As the bond order increases, the stability of the bond increases. Molecules with high bond order are more stable and less reactive. Molecules with low bond order are less stable and highly reactive. If the bond order is zero, such a molecule does not exist.
Bond order also describes the magnetic properties of a molecule. If the bond order is a positive integer, the molecule is diamagnetic. If it is a decimal number, it is paramagnetic.
Usually, bond order 1 is a single bond, 2 is correlated with a double bond and 3 is correlated with a triple bond.

The cover image was created using an image by Tomislav Jakupec from Pixabay