More results...

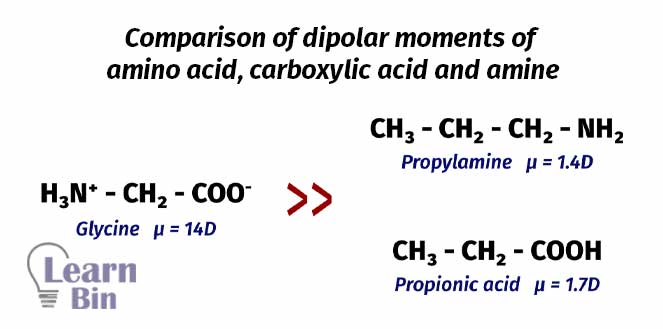
Usually, amino acids are very stable species. They have high melting points of over 200℃. Due to the polarity of amino acids, they are highly soluble in polar solvents like water. Amino acids have charged around. So, they show high dipolar moments than simple amines and simple carboxylic acids.
Amino acids have both carboxyl group (acidic) and amine (basic) functional groups. So, they have both acidic and basic properties. Such compounds are called amphoteric compounds. Depending on the pH of the medium, the predominant form of the amino acid can be changed. However, amino acids are less acidic than carboxylic acids and less basic than most amines.
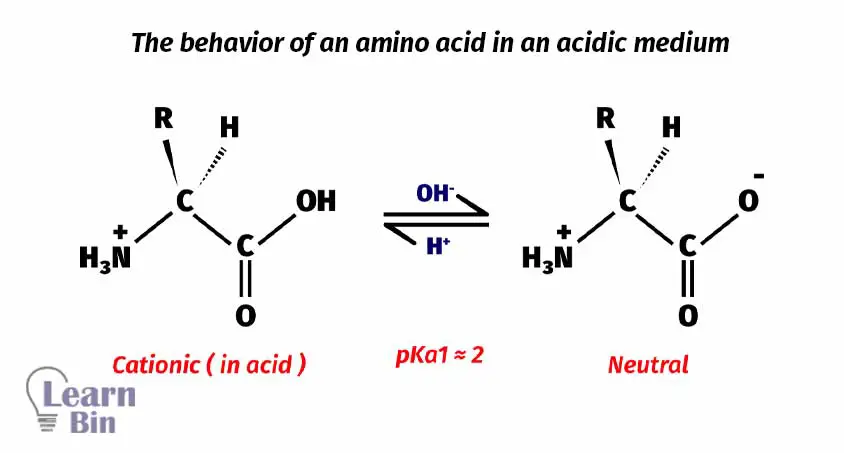
In acidic medium amino acids will be protonated. Therefore, the amino acid remains as a cation in an acidic medium.
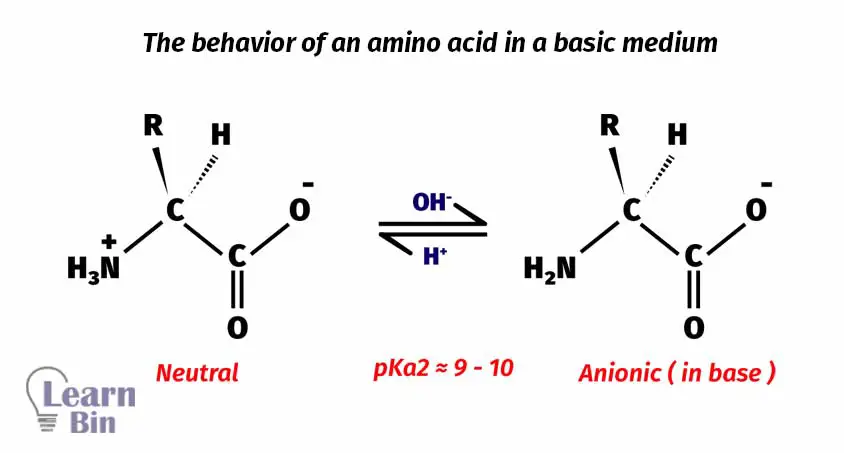
In the basic medium, amino acids will be deprotonated. Therefore, the amino acid remains an anion in the basic medium.
As an example for describing the acid-base properties of amino acids let's consider glycine. Glycine (the simplest amino acid) remains as a cation below pH = 2.3. below this pH, the amine group in glycine has been protonated. Therefore, it is positively charged.
When increasing the pH above 2.3, the carboxyl group will be deprotonated. So, there will be a negative charge on the carboxyl group. At this point, the amine group is positively charged and the carboxyl group is negatively charged. Therefore, the net charge on amino acid is zero. The molecule has charges on it but the overall charge is zero and these molecules are called zwitterions.
Glycine will remain as a zwitterion between pH = 2.3 and pH = 9.6. when pH is increased higher than 9.6, the protonated amine group will be deprotonated. Therefore, the positive charge on the amine group will be neutralized. At this point, there is a negative charge on the carboxyl group. So, the net charge of the amino acid molecule will become negative. In other words, the amino acid will remain as an anion.
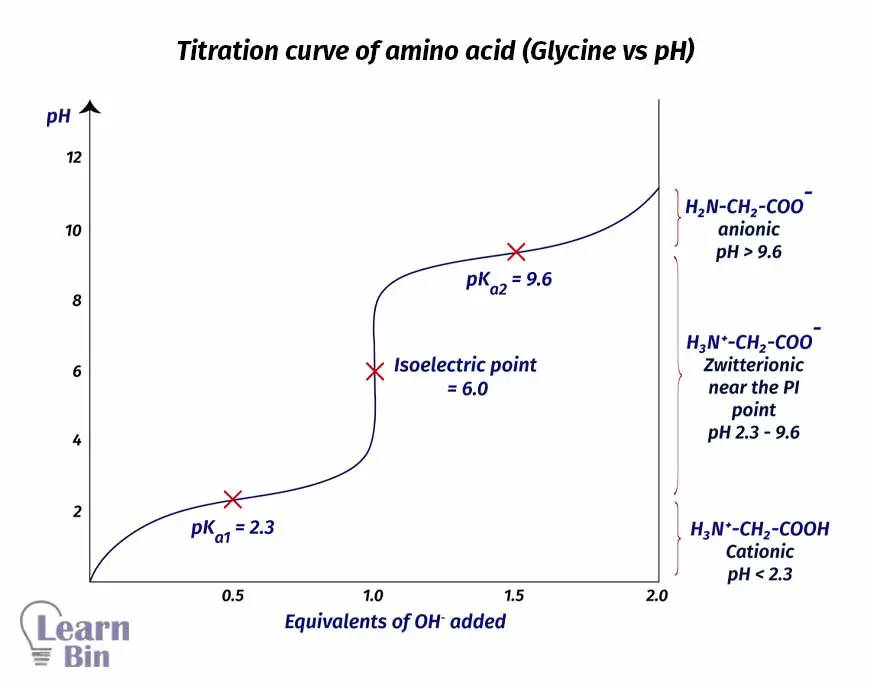
The isoelectric point is the pH at which the amino acid exists largely in a neutral or zwitterionic form. Due to the zwitterionic nature of amino acids, they possess isoelectric points (pI). The isoelectric point is influenced by the nature of the sidechain.
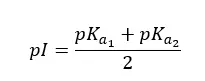
A zwitterion is a dipolar ion having both formal positive and formal negative charges. But the overall charge is neutral.
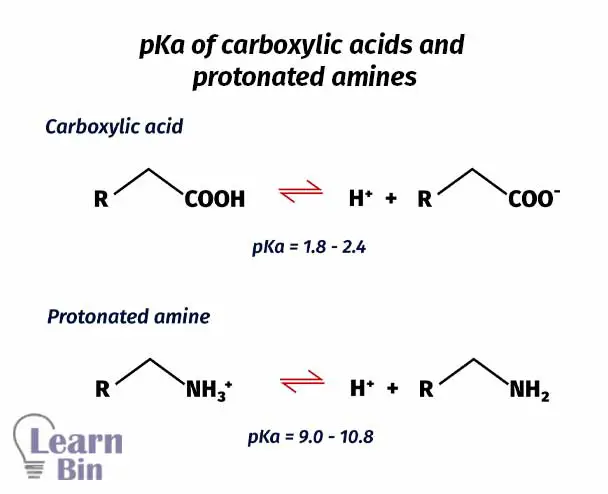
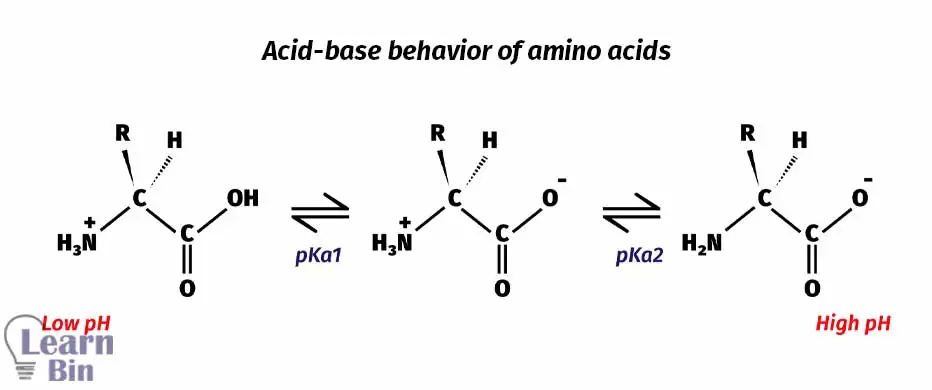
The above two reactions explain the two pKa values in amino acids. Lower the pKa value means higher acidity. So, the acidity is higher in carboxylic acid than in a protonated amine.
If the medium pH is lower than the first dissociation step, the molecule will be positively charged. If the medium pH is higher than the second dissociation step, the molecule has a negative charge on it. When the medium pH is in between the first and second dissociation steps, the molecule has no net charge (neutral).
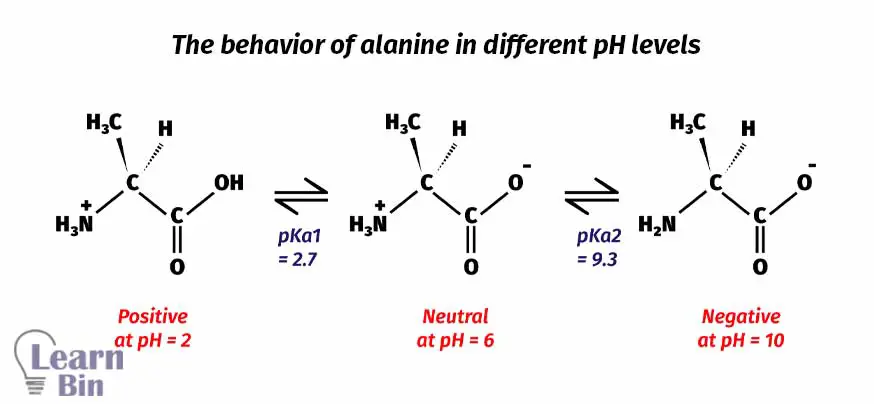
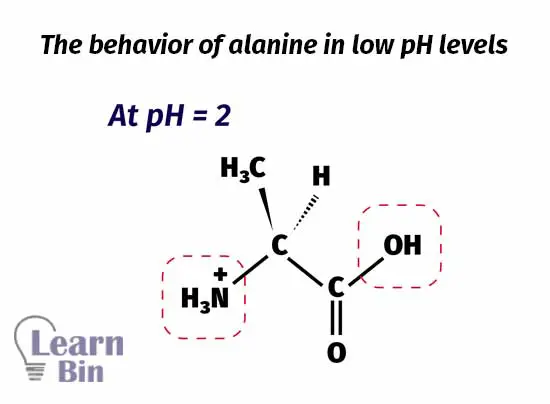
At pH = 2, the medium pH is not strong enough to deprotonate neither the carbonyl group nor the amine group. The Amine group is protonated. So, the amine group has a positive charge on it. Therefore, the molecule remains positively charged.
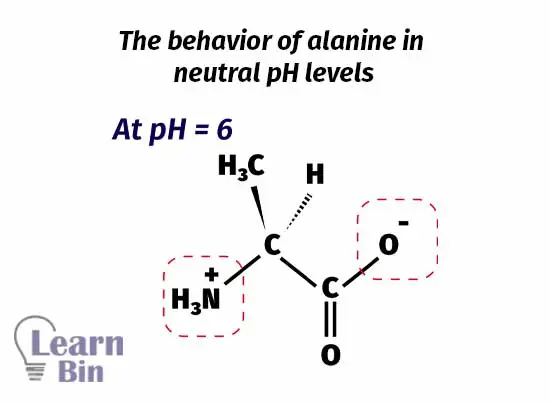
When increasing the pH of the medium, at pH = 6, the medium pH is strong enough to deprotonate the carboxylic group. But this pH is not enough to deprotonate the protonated amine group. At this point, the amine group has a positive charge on it because it has been protonated. Due to the deprotonation of the carboxyl group, there is a negative charge on it. But the molecule remains neutral because the overall charge is zero.
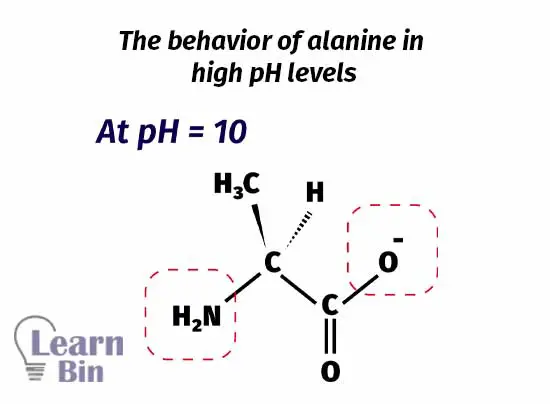
When increasing the pH of the medium further, at pH = 10 the protonated amine group is also deprotonated. At this pH, the carboxyl group has a negative charge on it. Therefore, the molecule remains as negatively charged.
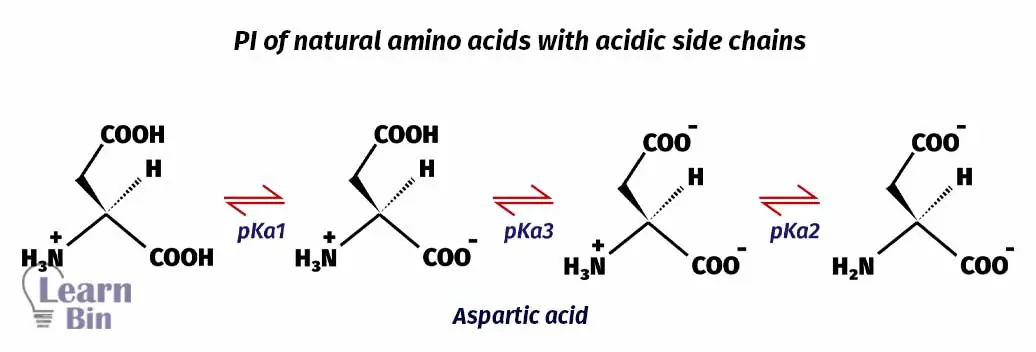
In amino acids with acidic side chains, pKa1 is where the carboxyl group is deprotonated. pKa2 is where the carboxyl group of the side chains is deprotonated. And pKa3 is where protonated amine group is deprotonated. Here, pKa1 and pKa3 are responsible for keeping the molecule neutral. (Between pKa1 and pKa3 molecule stays neutral). Therefore, the pI of amino acids with acidic side chains would be,
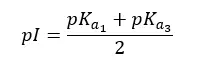
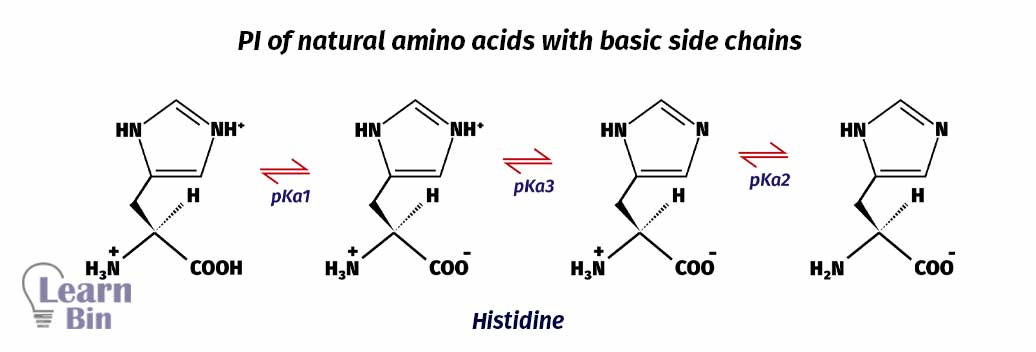
At lower pH, both the amine group and basic side group have been protonated. In amino acids with basic side chains, pKa1 is where the carboxyl group is deprotonated. pKa2 is where the amine group is deprotonated. And pKa3 is where protonated -NH group of basic side chain deprotonated. Here, pKa2 and pKa3 are responsible for keeping the molecule neutral. (Between pKa1 and pKa3 molecule stays neutral). Therefore, the pI of amino acids with basic side chains would be,
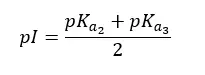

The cover image was created using an image by DCStudio on Freepik