More results...


In the manufacturing industry, raw materials are converted into valuable products chemically, physically, biologically, or in any other combination. Here, the raw materials are subjected to a series of events. This overall series of events is known as a Process.
This process can be chemical, physical, mechanical, or a combination of them. A typical process consists of individual processing units. The individual processes happening in each individual process unit are called “Unit operation”.
To get a better idea of the process, process units, and unit operations, let’s consider the following example.
| Process | Process unit | Unit operation |
| Preparation of chocolate chip cookie dough | Mixer | Mixing |
In this example, the preparation of chocolate chip cookie dough is a sub-process of manufacturing chocolate chip cookies. The entire process may have different process units and unit operations.
There are three main types of processes in the industry based on the input of materials and output of the products.
In a batch process materials are filled into the 'process unit'. After the materials are processed, they will be discharged. The process is uninterrupted while processing materials.
Materials are entered, and processed, and products are removed from the process continuously. This system is in a steady state. This type of process needs to shut down after periods of time.
Here, processing materials are continuously happening. But some materials are entering, and products are discharging at intervals of time.
Chemical reactors are a good example of process units used in industries. There is a more detailed article about chemical reactors and you can learn more about process units. Also, you can read more about common process units used in plastic processing using this link.
Also, You can find out more examples of process units in the below section about unit operations.
In a process, there can be different types of unit operations.
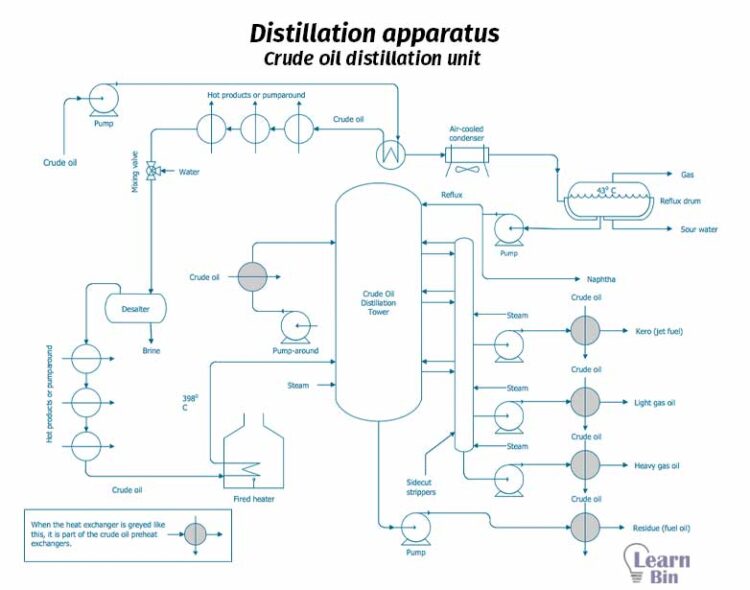
Distillation is a separation technique for the separation of liquids. When there is a mixture of liquids with different boiling points, the distillation method is used to separate them. A such mixture is heated, the liquid with the lowest boiling point (more volatile component) starts to boil when the temperature reaches its boiling point. At that point, that liquid is converted to vapor. Therefore, the vapor phase is rich in the more volatile component. This vapor is separated and condensed.
Liquid with a high boiling point (less volatile component) remains in the liquid phase. this process is simply known as “Distillation”. Distillation is an equilibrium stage operation and physical separation technique.
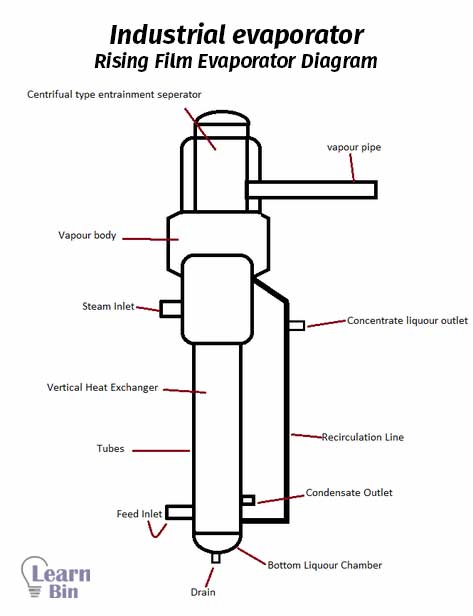
Evaporation is a physical separation technique that separates non-volatile components from volatile components in a solution. As an example, in the salt industry, water is evaporated to separate salt. When evaporating the volatile component, the concentration of the non-volatile component in the solution is increased.
E.g.:-
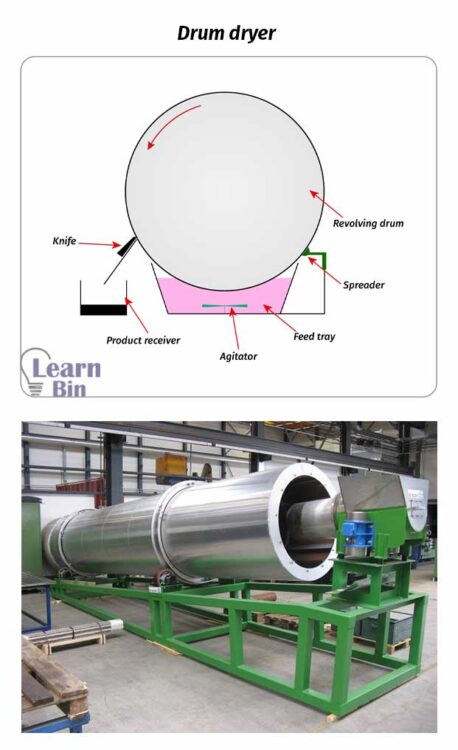
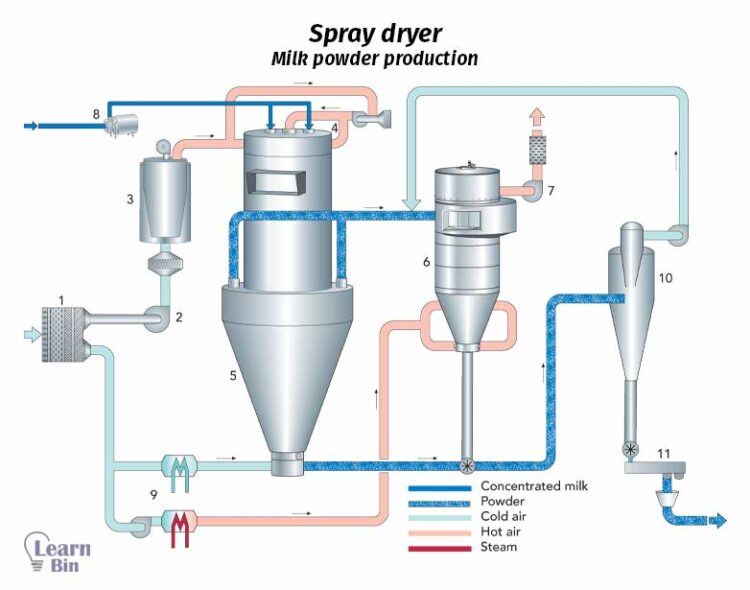
If there is a small amount of volatile components remaining in solid materials, those volatile components are removed by applying heat in the drying process.
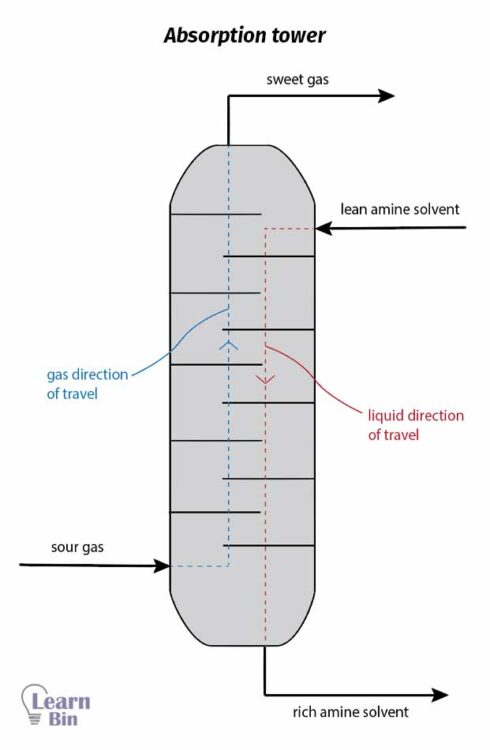
A component in a gas phase is transferred to a liquid phase. For the absorption process, absorption towers are used in industry. In an absorption tower, solute-rich gas is entered at the bottom, and the solvent is entered at the top of the tower. When passing through the tower gas phase and the liquid phase are contacting each other. So, the solute is transferred to the liquid phase. A solute-rich solvent is collected at the bottom of the tower.
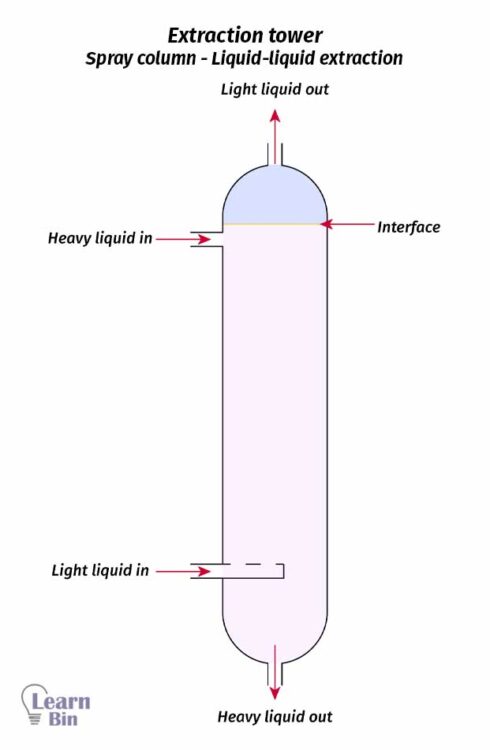
A solute dissolved in a liquid solution is transferred to another liquid solvent. Here, solute should be dissolved in both liquids and two liquids should be immiscible.
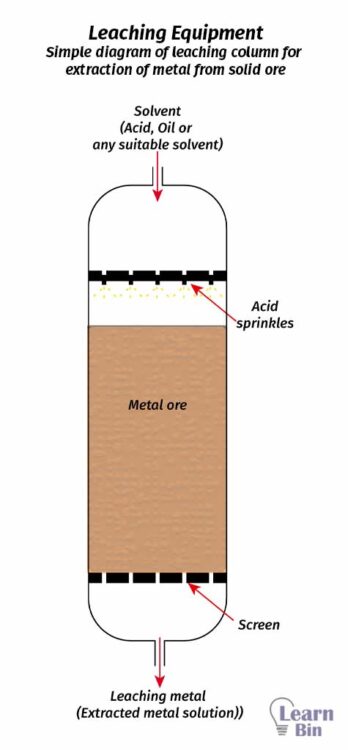
The solute is extracted from a solid, using a solvent. A fluid is treated with a finely divided solid. So, the solute in the solid will be extracted from the solvent.
E.g.:–
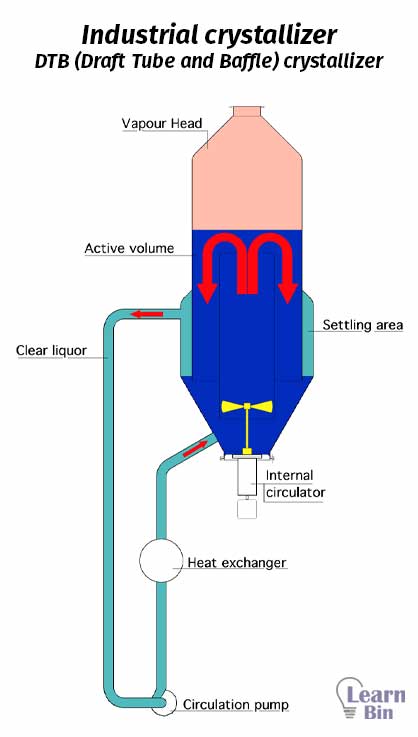
Dissolved solids are separated from a solution, vapor, or melt in crystallization unit operation. In this process, pure crystals are formed at low energy.
E.g.:-
In this unit operation, components in a gas stream or a liquid stream are adsorbed to a solid adsorbent. This process is mainly used in wastewater treatment and waste gas treatment.
E.g:-
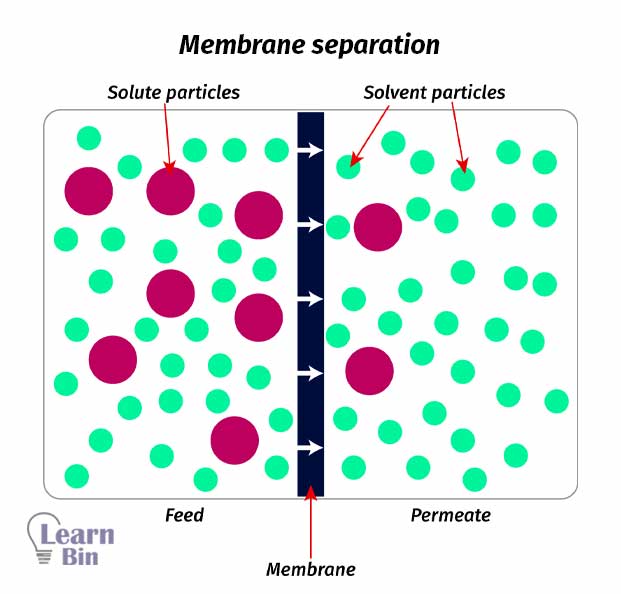
Solutes are separated from a solution using a semi-impermeable membrane. When passing the solution through the membrane, solvent particles will pass through the membrane. And the membrane is a barrier to solutes.
E.g:-
Solid particles in a liquid or gaseous fluid are removed using a screen or cloth. a pressure difference is set up and causes the fluid to flow through the filter. Large solid particles in the fluid will remain on the filter. Small fluid particles will flow through the filter.

The cover image was designed using an image by Ekkasit Chaingam from Pixabay
Figure 01: Contains an image by CS Odessa, licensed under CC BY 4.0, via Wikimedia Commons
Figure 02: Contains an image by Jasminenoh, licensed under CC BY-SA 3.0, via Wikimedia Commons
Figure 03: Contains an image by De Boer, licensed under CC BY-SA 4.0, via Wikimedia Commons
Figure 04: Contains an image by Shalini Srinivasan, licensed under CC BY-SA 4.0, via Wikimedia Commons
Figure 05: Contains an image by Jocelynskim, licensed under CC BY-SA 4.0, via Wikimedia Commons
Figure 08: Contains an image by Ruben Castelnuovo, licensed under CC BY-SA 3.0, via Wikimedia Commons