More results...


Lipids are heterogeneous compounds that are basically composed of Carbon, Hydrogen, and Oxygen. Every lipid is insoluble in water but they all are soluble in organic solvents. Lipids are formed by fatty acids and alcohol. Lipids can be classified in many ways.
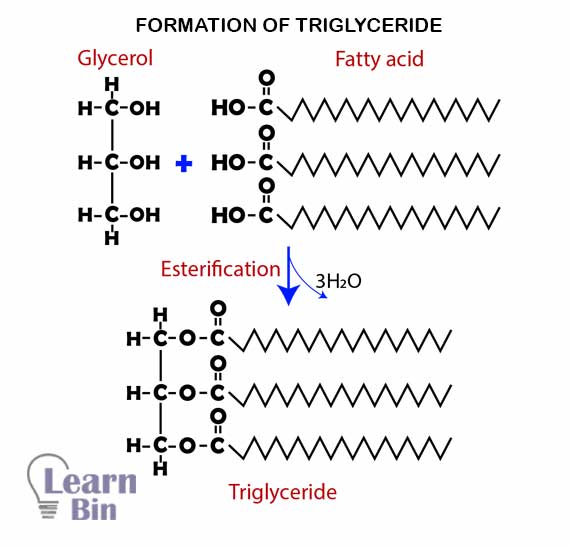
Simply lipids are formed by bonding three fatty acids with a glycerol molecule by ester bonds. Glycerol is an alcohol that has three carbon atoms and three hydroxyl groups. (C3H5(OH)3). Fatty acids are hydrocarbon chains that contain a hydroxyl group (-COOH). There are two types of fatty acids.
These types of lipids are called "Triglycerides".
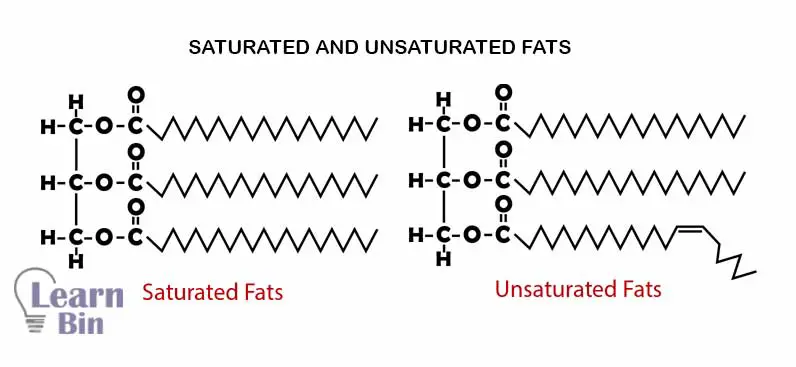
There are only single bonds between carbon atoms of the hydrocarbon chain of the fatty acid. Lipids that are formed from saturated fatty acids are called “saturated lipids” Saturated lipids have relatively high melting points and boiling points.
The general formula for saturated fatty acids - CnH2n+1COOH
There are both single bonds and double bonds between carbon atoms of the hydrocarbon chain of the fatty acid. Lipids that are formed from saturated fatty acids are called “unsaturated lipids” saturated lipids have relatively low melting points and boiling points. The saturation and the number of carbon atoms in the hydrocarbon chain are directly affected by the melting points of fatty acids.
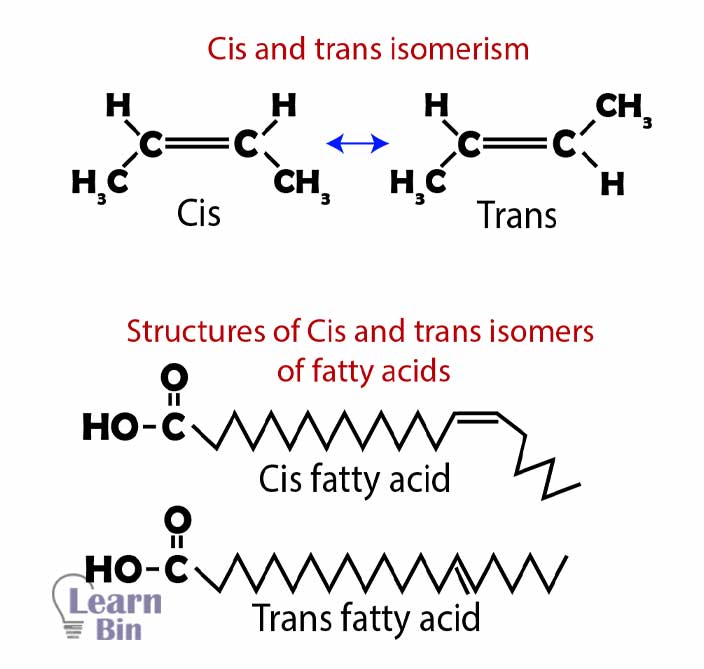
Fatty acids with the same number of carbons and double bonds form cis and trans structures and those are not identical. Cis fatty acids are predominant and trans fatty acids exist in small quantities naturally.
Lipids which are liquids at room temperature are called oils. Mainly, oils are made out of unsaturated fatty acids. Oils can be found as storage food in plants. In seeds, nuts, and cereals oils can be found as storage food.
E.g.:-
Lipids that are solids at room temperature are called fats. Mainly, fats are made out of saturated fatty acids. The main storage food in animals is fat.
E.g.:-
The melting point of lipids is controlled by mainly the number of carbon atoms and the degree of unsaturation in the fatty acid chain. Lipids with long-chain fatty acids have higher melting points than lipids with short-chain fatty acids. Because the increasing length of the fatty acid chain caused the lipid to be crystallized.
On the other hand, with the increase in the degree of unsaturation (amount of double bond between carbon atoms), the melting point is decreasing. Because of the unsaturation, the lipids do not tend to be crystallized. So, lipids with long chains and saturations form crystals and have high melting points (fats). And the lipids with short chains and a higher degree of unsaturation have low melting points (oils)
Fats do not have sharp melting points. They gradually soften or melt on heating. When heated to higher temperatures fat begins to smoke (smoke point) then ‘flashes’ (flashpoint) and finally burns (fire point). These temperatures are important when selecting an oil for frying purposes in the food industry.
The viscosity of fat is caused by the internal friction between the molecules. As the number of molecules that make up fat increases, the viscosity increases. So, lipids with short-chain fatty acids have higher viscosities.
Increasing the degree of saturation decreases the viscosity, and as the length of the fatty acid chain increases, the viscosity of the lipid decreases.
Fats and oils have a lower density than water. Approximately it is between 700 kg/m3 - 900 kg/m3. At the solid state fats/oils have high densities. When it melts the volume increases, so the density decreases.
All the fats and oils are not dissolved in water but dissolved in organic solvents such as hydrocarbons or alcohol.
In addition to alcohol, and fatty acids there are other chemical groups in complex lipids. Phosphate groups, sugar, and nucleosides can be found in complex lipids.
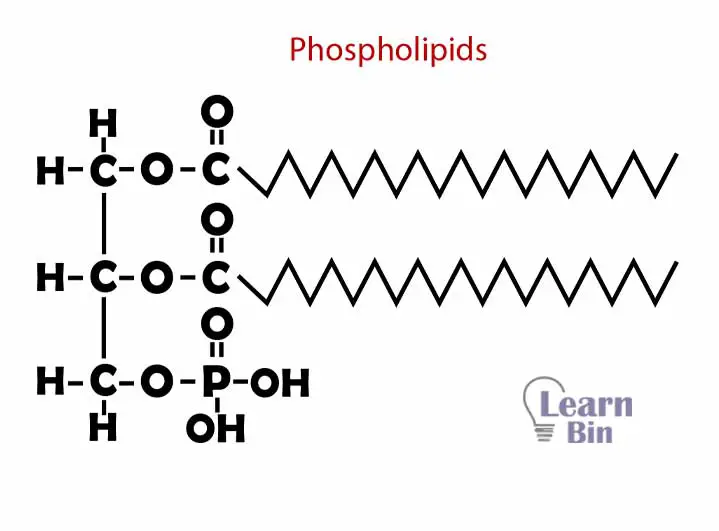
Phospholipids are formed by glycerol, fatty acids, and a phosphate group. A glycerol group is attached to two fatty acids and a phosphate group. Glycerol and phosphate part has polar properties (hydrophilic) and two fatty acid chains have nonpolar properties (hydrophobic). Phospholipids are the main structural component in the cell membrane.
Glycolipids are formed by carbohydrates and lipids. Carbohydrates are attached to the lipid part by glycosidic bonds. Glycolipids can be found on the surface of all eukaryotic cell membranes. Glycolipids are extended from the phospholipid double (on the cell membrane) layer to the extracellular environment. The role of glycolipids in the cell membrane is to maintain cell membrane stability and facilitate cell identification. Because they are important for the immune response and for cells to connect to each other and form tissues.
Lipoproteins are a type of biochemical that is formed by lipids and proteins.
Waxes are formed by fatty acids and alcohols that are not glycerol. Generally, all waxes are at solids at room temperature. Waxes consist of long-chain fatty acids and long-chain alcohols. Due to their severe rabies nature, they can act as water repellents on the leaves of some plants, feathers, and cuttings of some insects.
Fats are more palatable and storable to unlimited compared to carbohydrates. Fats and oils are respiratory substrates in animals. And fats are stored in fat tissues and it is called depot fats. They have high energy value (9 kcal) than carbohydrates (4 kcal) and proteins (4 kcal). And also, stored lipids act as a thermal insulator layer in the body and it is important in heat regulation in the body.
Some lipids contribute to building the structure of cells such as phospholipids in the cell membrane. All lipids are insoluble in water, which helps in the conservation of water in living organisms. (waxes) lipid helps the body to absorb fat-soluble vitamins which are vitamins A, D, E, and K.

Healthline. Saturated vs. Unsaturated Fat: Know the Facts.
Abasolo, C . Physical properties of oils and fats - BTSA.
Soffar, H, Complex Lipids function, definition, types & structure, Phospholipids & Glycolipids | Science online.
Fao.org. Chapter 3: Calculation of the energy content of foods - Energy conversion factors.
Cover Image by Steve Buissinne from Pixabay