More results...


Acids and bases can be simply explained according to their ability to donate H+ or OH- ions. There are few theories that describe what acids and bases are.
According to Arrhenius theory acids are chemical substances that dissociate in water and donate H+ ions to the medium. Bases are chemical substances that dissociate in water and donate OH- ions to the medium.
According to the Bronsted Lowry theory acid is any substance that has the ability to donate H+ ions. And bases are substances that have the ability to donate OH- ions.
According to the Lewis theory chemical substances that contain empty orbital and are capable of accepting electron pairs are known as Lewis acids. Lewis bases are substances that will donate electron pairs.
OH- ions can donate electron pairs. Therefore, OH- is a Lewis base. H+ accepts electron pairs. Therefore, H+ is a Lewis acid. According to the Lewis theory NH3, H2O, CN-, etc are Lewis bases. AlCl3, BCl3, and BeCl3 are examples of Lewis acids.
Acids are substances that donate H+ ions or accept electron pairs.
In an aqueous medium, strong acids will dissociate completely and donate H+ ions to the medium.
E.g,
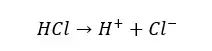
But weak acids will not dissociate completely. They will dissociate partially, and the dissociation reaction is reversible. Therefore, there are undissociated acid molecules and dissociated ions in a weak acid solution.
e.g.,
Let’s consider a weak acid, HA. It will dissociate in the aqueous medium as follows.
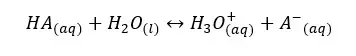
We can write the equilibrium constant for the concentration for the above reversible reaction as follows.
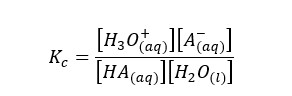
In the above equation, the concentration of the water remains constant. So, we can write a new constant as Ka as follows.
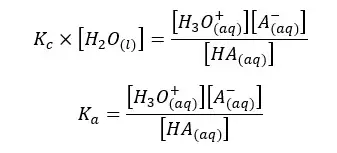
Ka is known as the dissociation constant of the acid. It only depends on the temperature. According to the Ka value of a particular acid, we can predict whether the acid is strong or weak. If the dissociation constant of an acid is very high, that means the acid is strong.
When the Ka value decreases, the acidic strength is also decreasing. Acids with Ka values larger than 1 are considered strong acids. Acids with Ka values less than 1 are considered weak acids.
Bases are substances that donate OH- ions to the medium or donate electron pairs.
Strong bases will completely dissociate in an aqueous medium and donate OH- ions to the media.
e.g.
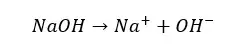
Weak bases are bases that will dissociate partially in an aqueous media. The dissociation of a weak base is reversible. In a weak base solution, both undissociated base molecules and dissociated ions can be found at once.
e.g
Let’s consider a weak base, B. It will dissociate in the aqueous medium as follows.
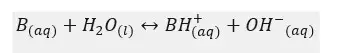
Let’s write the equilibrium constant of concentration for the above equilibrium.
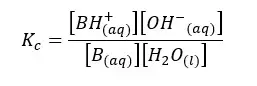
In the above equation, the concentration of the water remains constant. Therefore, for both Kc and [H2O] constants, we can write a single constant as Kb as follows.
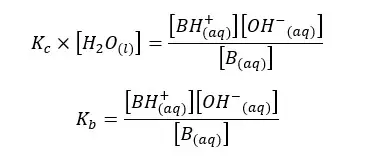
Kb is the dissociation constant of the base. As same in acids, we can predict the basic properties of a base according to the Kb value.
If the kb value is higher than 1 that means the base is strong. If the kb value is less than 1, the base is weak. Kb only depends on the temperature.
Even pure water can conduct electricity. To conduct electricity there should be free electrons or free ions in the media. Therefore, even in pure water, there should be ions that are responsible for conducting electricity. Pure water can be ionized as follows,
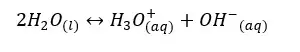
or
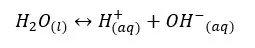
Let’s write the equilibrium constant of concentration for the above equation.
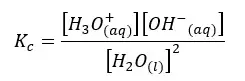
As the concentration of the water remains constant, we can write a new constant as Kw as follows.
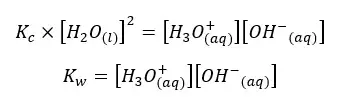
This constant is known as the ion product of water. At 25℃, the ion product of the pure water is 1.01×10−14 mol2 dm-6. Kw only depends on the temperature. When increasing the temperature, the dissociation is increased. Therefore, the ion concentration also increased. Hence the Kw is increased.
Let’s find the concentrations of H+ ions and OH- ions of pure water at 25℃.
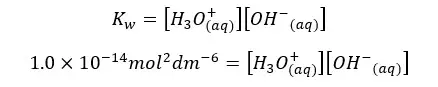
Since the concentrations of H+ ions and OH- ions are equal. Let’s take it as “x”. so, the above equation can be written as follows.
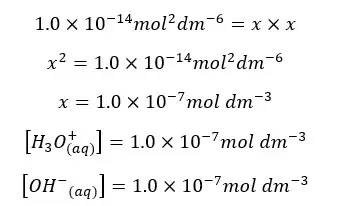
In pure water the concentrations of H+ ions and OH- ions are equal. When changing the temperature ion concentrations will be changed. But at any temperature, the concentration of H+ ions and OH- ions will be equal in pure water.
If the concentration of H+ ions is higher than OH- ions, the water is acidic. If the OH- concentration is higher than the H+ ions, the water is basic. Pure water is neutral.
pH value is used to express the acidic, basic properties of water.
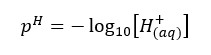
Where [H+] is the hydrogen ion concentration of the water. As same in the pH value, the pOH value is expressed using the concentration of OH- ions in the water.
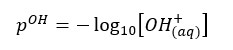
Most of the calculations included in the below sections are basic calculations that can use to find the pH value that is closer to the actual value. But to find a more accurate answer, we have to use some more advanced calculations. You can find out more advanced calculations that can be used to find more accurate answers for the pH in this article.
Problem 01
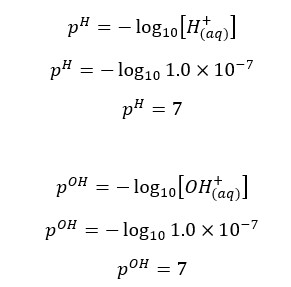
At 25℃, pH and pOH are 7.
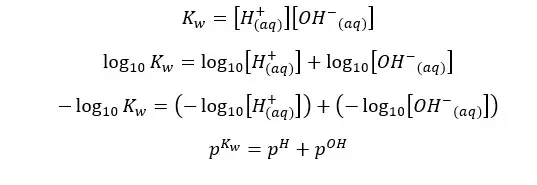
At 25℃,
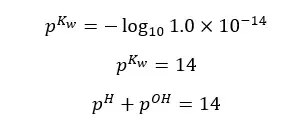
Although the concentration of H+ ions and OH- ions change, the sum of pH and pOH equals 14. (at 25℃)
Problem 02
Hydrochloric acid will completely dissociate and give H+ ions to the water. Water molecules in the medial also partially dissociated and give H+ ions to the medium.
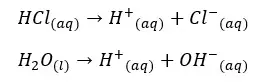
H+ ions that are given by the dissociation of HCl = 0.1 mol dm-3
H+ ions given by the partial dissociation of water = 1 × 10-7 mol dm-3
Compared to the H+ ions given by HCl, H+ ions from the dissociation of water is negligible. Therefore, we take the concentration of H+ ions given by HCl, as the total concentration of H+ ions.
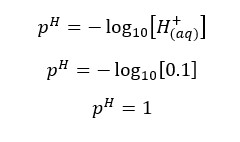
In this situation also the H+ ions from the dissociation of water are negligible. Therefore, we take only the H+ ions which are given by the dissociation of HNO3 acid.
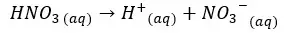
Concentration of H+ ions = 0.01 mol dm-3
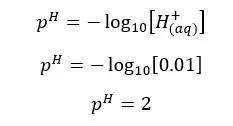
H2SO4 will dissociate as follows. H+ ions given by the dissociation of water are neglected here.
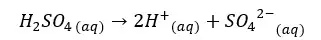
Since one H2SO4 molecule gives two H+ ions to the media, the concentration of H+ ions will be = 0.05 mol dm-3
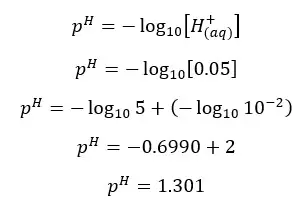
In his situation, the H+ ions given by the dissociation of water are not negligible.
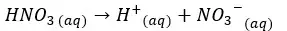
H+ ions given by the dissociation of water = 1.0 × 10-7 mol dm-3
H+ ions given by the dissociation of HNO3 = 1.0 × 10-8 mol dm-3
Total H+ ion concentration = 1.1 × 10-7 mol dm-3
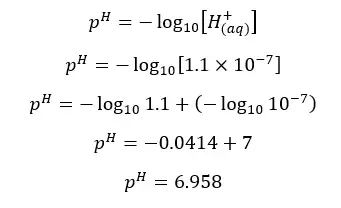
Problem 03
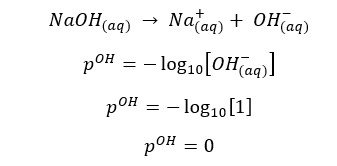
OH- ions given by the partial dissociation of water have been neglected here.
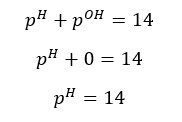
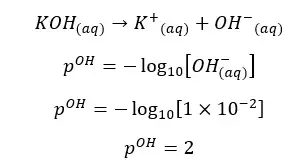
OH- ions given by the partial dissociation of water have been neglected here.
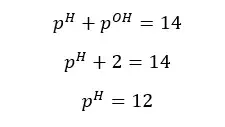
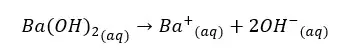
Concentration of OH- ions = 2 × 0.009 mol dm-3 = 0.018 mol dm-3
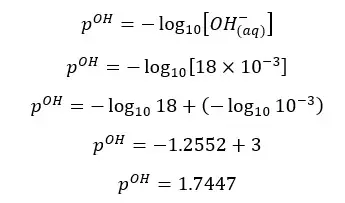
OH- ions given by the partial dissociation of water have been neglected here.
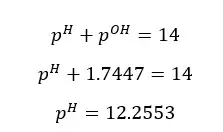
Since the concentration of NaOH is very low, the OH- ions given by the partial dissociation of water cannot be neglected here.

Let’s consider a monoprotic weak acid of HA with an initial concentration of C mol dm-3 and the dissociation of α mol dm-3. The acid will be partially dissociated as follows.
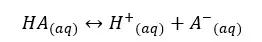
The concentrations of each component can be expressed as follows.
| Component | HA | H+ | A- |
| Initial concentration | C | 0 | 0 |
| Concentration after dissociation | C(1 - α) | Cα | Cα |
α is the dissociation of the acid per 1 mol. If we have an acid solution with 1 mol dm-3 concentration, the concentration of the acid after the dissociation would be 1- α.
Since we have a C mol dm-3 solution, we should multiply 1- α by C, to get the concentration after dissociation. We can write the Ka expression for the above equilibrium as follows.
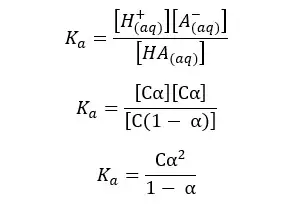
When compared to 1, α is very small. Therefore, 1- α is approximately equal to 1. So, the above expression can be recreated as follows,
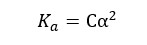
Where,
If we neglect the H+ ions given from the partial dissociation of water, the concentration of the H+ ions would be Cα. So, the pH of the media can be found as follows,
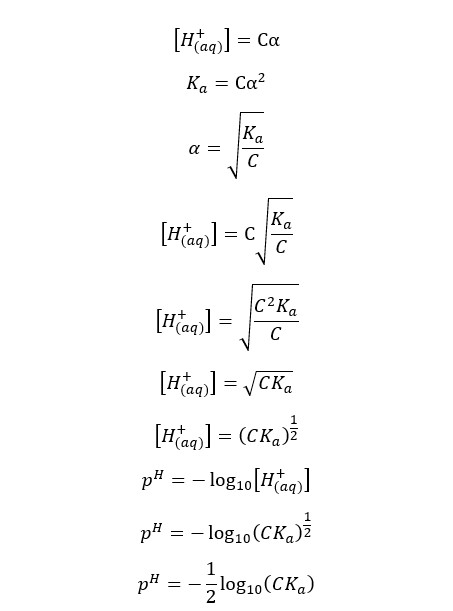
Since the summation of pH and pOH is 14 at 25℃, we can find the pOH of the weak acid as well.
Ostwald’s dilution law states that the dissociation of a weak acid is inversely proportional to the concentration of the acid at a given temperature.
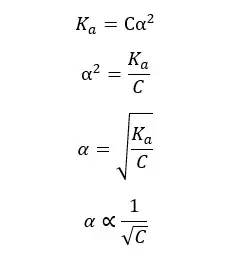
Problem 04
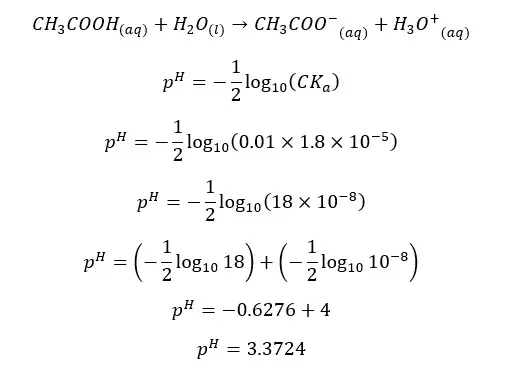
Let’s consider a monoprotic weak base of BOH with an initial concentration of C mol dm-3 and the dissociation of β mol dm-3. The base will be partially dissociated as follows.
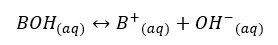
The concentrations of each component can be expressed as follows.
| Component | BOH | B+ | OH- |
| Initial concentration | C | 0 | 0 |
| Concentration after dissociation | C(1- β) | Cβ | Cβ |
β is the dissociation of the base per 1 mol. If we have a base solution with 1 mol dm-3 concentration, the concentration of the base after the dissociation would be 1 - α.
Since we have a C mol dm-3 solution, we should multiply 1 - α by C, to get the concentration after dissociation. We can write the Kb expression for the above equilibrium as follows.
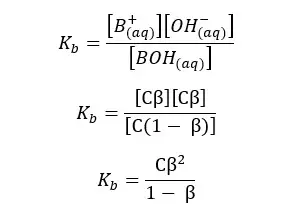
When compared to 1, β is very small. Therefore, 1 - α is approximately equal to 1. So, the above expression can be recreated as follows,
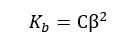
Where,
If we neglect the OH- ions given from the partial dissociation of water, the concentration of the OH- ions would be Cβ. So, the pOH of the media can be found as follows,
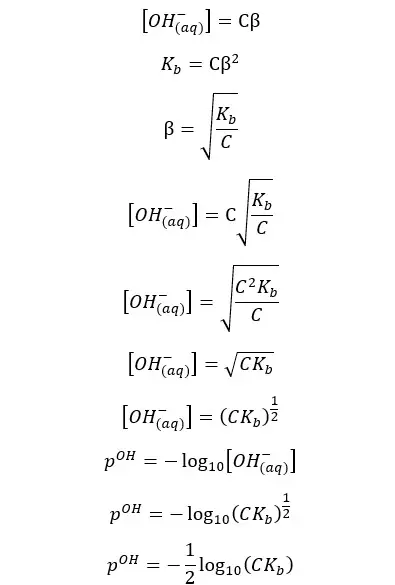
Since the summation of pH and pOH is 14 at 25℃, we can find the pH of the weak acid as well.
Problem 05
CH3NH2 will partially dissociate in the water as follows,
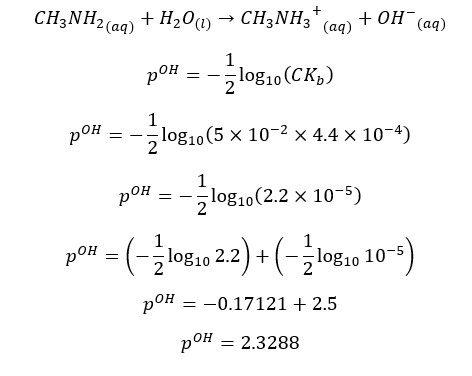
At 25℃,
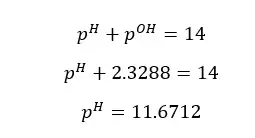
According to the Bronsted-Lowry definition of acids and bases, acids are proton donors and bases are proton acceptors. When an acid donated a proton, it will create an ion that is capable of accepting a proton. Since the proton acceptors are bases, that ion can be known as the conjugated base of the particular acid.
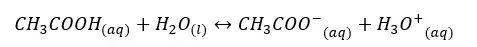
In the above equilibrium, H+ ions are donated in a forward reaction. Therefore, CH3COOH is an acid. In a backward reaction CH3COO- are accepting H+ ions and acts as a base. Therefore, CH3COO- is the conjugated base of the CH3COOH acid.
Bases accept protons. After accepting a proton, they will create ions that can donate a proton. That means an acid. That ion is the conjugated acid of the above base.
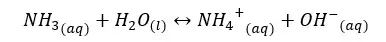
In the above equilibrium, H+ ions are accepted in a forward reaction. Therefore, NH3 is a base. In a backward reaction, NH4+ ions are donating H+ ions and act as an acid. Therefore, the NH4+ ion is the conjugated acid of the NH3 base.
In an aqueous media, acetic acid (CH3COOH) will dissociate as follows.
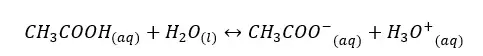
The forward reaction is a proton donor reaction. So, we can write the Ka expression for the forward reaction.
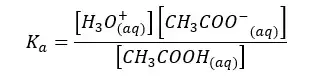
When water I added CH3COO- ion, the following hydrolysis reaction will occur. This reaction is similar to the back reaction of the above equilibrium.
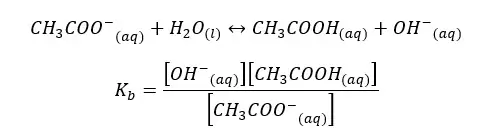
By multiplying the above Kb and Ka equations,
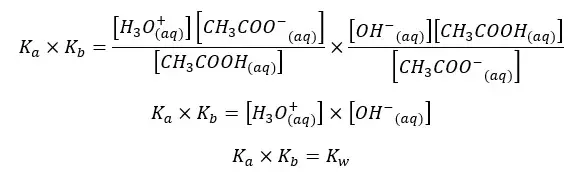
The above relation shows that the multiple of Ka and Kb of an acid and its conjugated base at a given temperature equals the ion product of water (Kw).
Problem 06
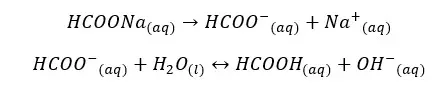
HCOONa salt will completely dissociate in water and result in HCOO- ions and Na+ ions in the media. HCOO- ions show an equilibrium with the water. let’s take α as the amount of dissociation at the equilibrium.
| Component | HCOO- | HCOOH | OH- |
| Initial concentration | 0.02 | 0 | 0 |
| Equilibrium concentration | 0.02 - α | α | α |
At 25℃,
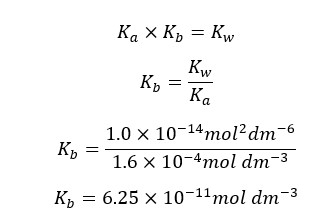
We can write the Kb expression for the above equilibrium.
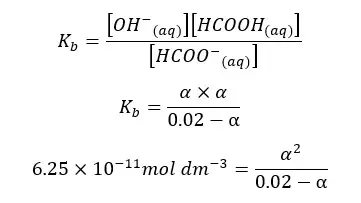
When compared to 0.02, α is too small. Therefore, 0.02 - α is approximately equal to 0.02. according to that, the equation will be recreated as follows.
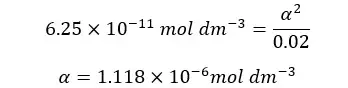
α is the OH- ion concentration of the media. Therefore, we can find the pOH of the media.
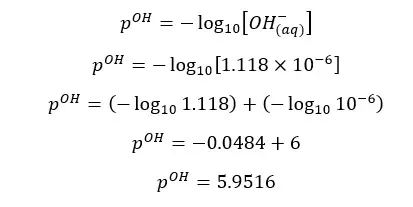
At 25℃,
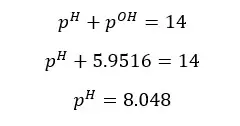
The pH of the above salt solution is greater than 7. That means the solution is basic. Salts which are resulted from reacting strong bases and weak acids are basic.
Problem 07
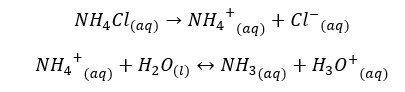
NH4Cl salt will completely dissociate in water and result in NH4+ ions and Cl- ions in the media. NH4+ ions will partially dissociate with NH3 and H+ ions in water. If β is the amount of dissociation at equilibrium, concentrations of each component would be as follows,
| Component | NH4+ | NH3 | H+ |
| Initial concentration | 0.02 | 0 | 0 |
| Equilibrium concentration | 0.02 - β | β | β |
At 25℃,
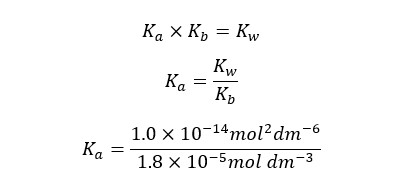
We can write the Ka expression for the above equilibrium.
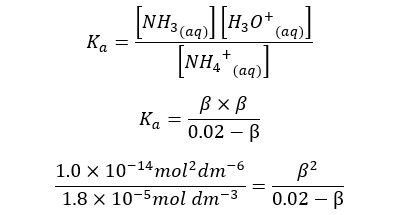
When compared to 0.02, β is too small. Therefore, 0.02 - β is approximately equal to 0.02. according to that, the equation will be recreated as follows.
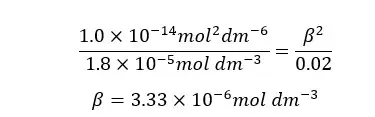
β is the H+ ion concentration of the media. Therefore, we can find the pH of the media.
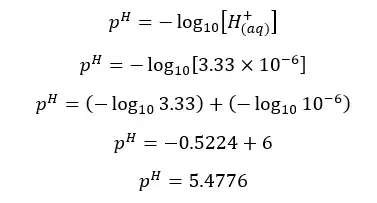
The pH of the above salt solution is less than 7. That means the solution is acidic. Salts which are resulted from reacting strong acids and weak bases are acidic. An aqueous solution of a salt is formed by reacting strong acid and a strong base is neutral. At 25℃ the pH of such solution is 7.
