More results...


Polymer morphology is the structure, arrangement, and physical form of polymer molecules. There are three types of polymer morphologies which are Amorphous, crystalline, and semi-crystalline.
In amorphous polymers, polymer molecules are randomly arranged, and they are highly entangled. Amorphous polymers are the most common polymer morphology.
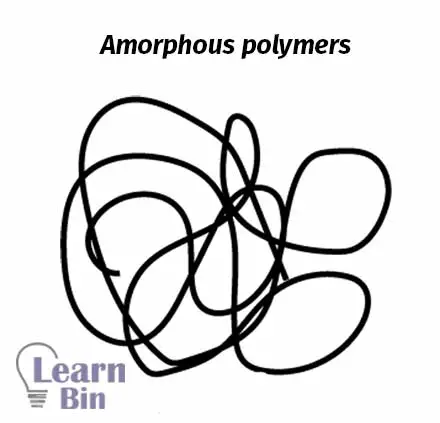
At low temperatures, amorphous polymers have a glassy texture. That means they are brittle and hard. As the temperature increases, at a particular temperature, the glassy texture becomes a rubbery texture. This temperature is called glass transition temperature. Every amorphous polymer has its specific glass transition temperature.
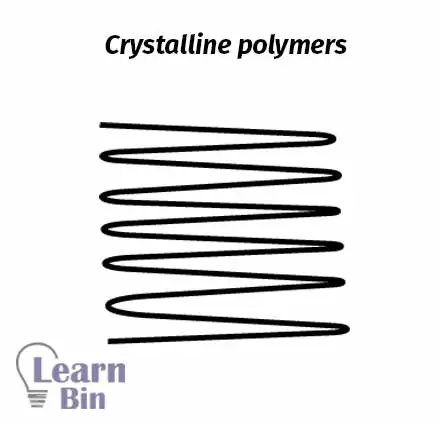
Polymer molecules in crystalline polymers are highly arranged in order. Crystalline polymers do not have glass transition temperatures, but they show transition as melting (Tm). The development of crystallinity depends on the regularity of the polymer structure.
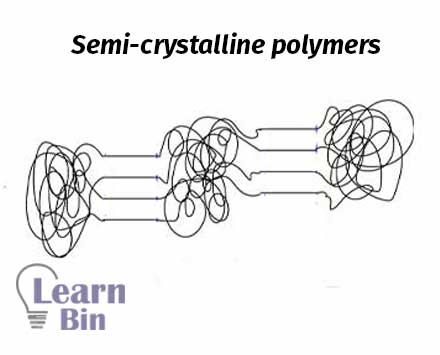
Most crystalline polymers are not entirely crystalline. There are some amorphous regions in the crystalline polymers. Some parts of the polymer chain are randomly arranged, and other parts are arranged in order. Therefore, semi-crystalline polymers have both amorphous and crystalline regions.
There are two important factors that affect the morphology of polymers. Which are polymer structure and intermolecular forces.
If the polymer molecule is regular and ordered, it will pack into crystals easily. There are three types of polymer tacticity which are isotactic, tactic, and syndiotactic. In isotactic polymers, pendent groups of all the repeating units have the same stereochemical configuration. In syndiotactic polymers, pendent groups in repeating units have alternating stereochemical configurations. Therefore, both isotactic and syndiotactic polymers get easily crystallized.
Atactic polymers do not have a regular stereochemical configuration of pendant groups in repeating units. So, atactic polymers tend to be amorphous. If there are any pendant groups in the polymer, it will affect the crystallinity of the polymer.
If a polymer hasn’t got any pendant groups, it still can be amorphous. Crystallinity also depends on the branching of the polymer. In polyethylene, there are no pendant groups but there is both crystalline and amorphous polyethylene.
Polyethylene with no branches is called linear polyethylene. Due to no branches, they can pack well easily. As well they have high density. So, they are crystalline, and they are called high-density polyethylene (HDPE). Branched polyethylene cannot be packed well, so they tend to be amorphous. The density is low in branched polyethylene, and they are called low-density polyethylene (LDPE).
There are several intermolecular forces between polymer molecules. Van der Waals forces are the most common type of intermolecular forces, and they are present in each and every polymer. Except that there are dipole-dipole forces and hydrogen bonding present in polymers. Dipole-dipole forces are present in polar polymers. Polymers with N, O, and H have hydrogen bonds. Hydrogen bonds help polymers to form crystals.
When processing the polymers, they have to be melted. After getting the desired shape the products allow being cool. The cooling process can be rapid or slow.
In the rapid cooling process, amorphous polymers are obtained. Because in a molten state, polymers are in the amorphous state.
Due to the cooling being rapid, polymer molecules have no time to arrange properly. So, they remain amorphous. In the slow cooling process, polymer molecules have enough time to arrange properly. So, in the slow cooling process, crystalline polymers are obtained.

It's really beneficial...how to get all this type of articles regularly to increase knowledge and refresh it?