More results...

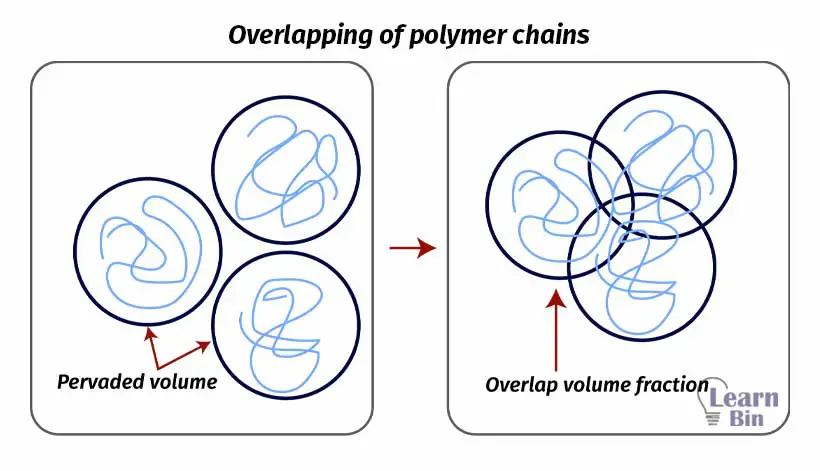
In dilute polymer solutions, polymer molecules will behave individually. But in polymer melts, polymer molecules will overlap each other. If the degree of polymerization (number of monomers in a polymer chain) is high or in other words there are longer polymer chains, polymer chains will be entangled. That means they will overlap more. The overlapping of polymer chains can be explained by the overlap parameter.
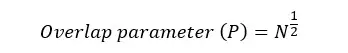
N is the degree of polymerization. When the degree of polymerization increases, the overlap parameter increases. Therefore, a higher overlap parameter means a higher level of entanglements. When the number of entanglements increases, the elasticity of the polymer increases.
Reptation theory describes the molecular motions of polymers in entangled polymer melts. In the Tube model, it is assumed that the motion of the polymer chain occurs in a hypothetical tube.
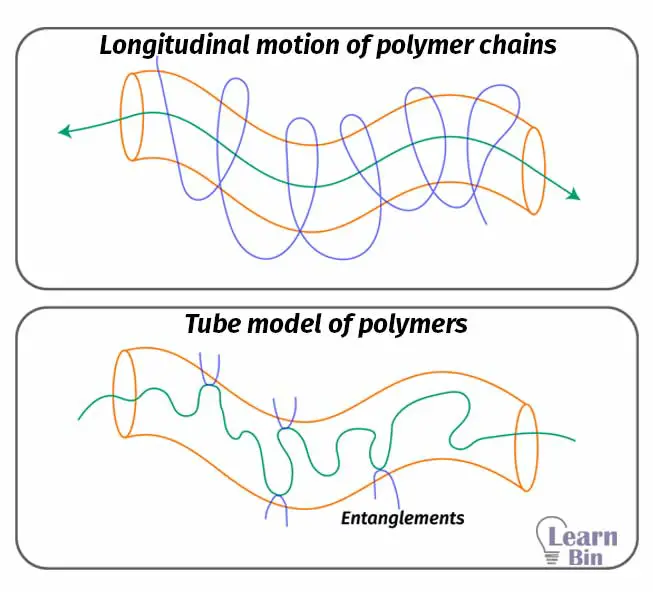
In a viscose polymer melt, the motion of an individual polymer chain is highly restricted by the surrounding polymer chains. This restricted domain almost looks like a tube. Polymer chains can move within that hypothetical tube. But the polymer can only move along the tube; not across. So, longitudinal motion is possible; not transverse. This motion is really like snake motion.
Brownian motion within the tube confinement is expressed using the diffusion coefficient of the tube. It is calculated using Einstein's relation. If the mobility of a polymer segment = μseg, the mobility of the polymer chain (tube) with N number of segments can be expressed as follows according to the tube model.
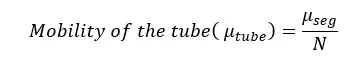
Using Einstein’s relation, the diffusion coefficient of the tube is given by,
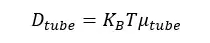
Where,
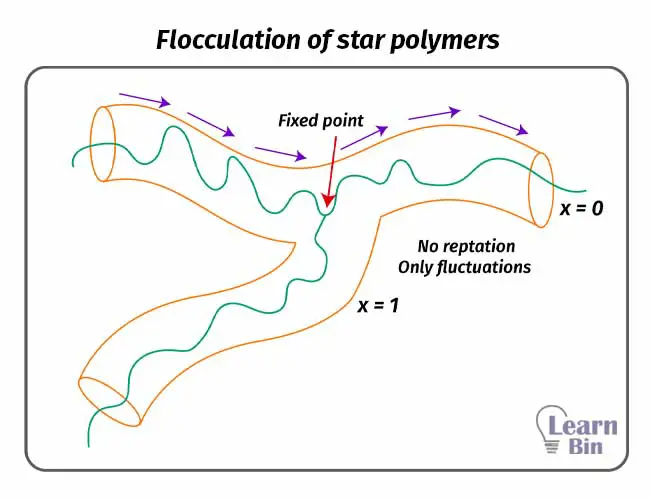
Reptation theory is mostly used for linear polymers. There is no Reptation in star polymers with fixed centers. Only flocculation occurred.
The tube model can be used to estimate the rheological properties of the polymer. For example, stress relaxation can be calculated as the fraction of the polymer chain that has been coming out from the original tube. If polymer chains are longer, they can make more entanglements and more flow-resistant. Therefore, they take a longer time to come out from the tube. That time is proportional to the viscosity of the medium.
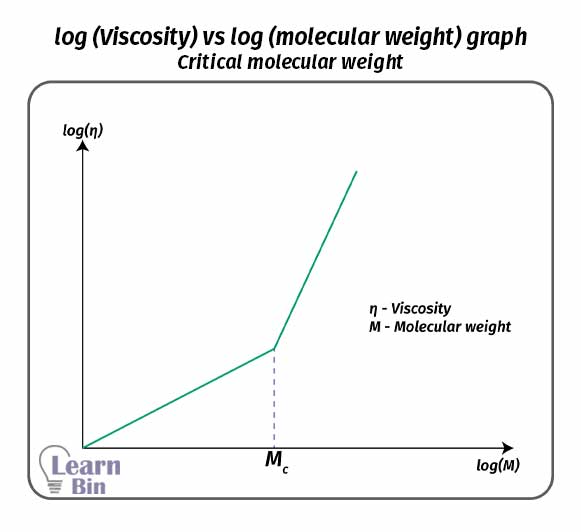
The critical molecular weight of a polymer is the minimum chain weight of the polymer for the formation of the entanglements. The number of entanglements is increased when the molecular weight exceeds the critical molecular weight. Therefore, the viscosity of the system also increased significantly after the critical molecular weight.
This value depends on the flexibility of the polymer chain. Above the critical value, the number of entanglements becomes sufficient to produce a strong rubber-like effect.
| Polymer | Critical molecular weight Mc (g mol-1) |
| polycarbonate | 4800 |
| Cis- polyisoprene | 10 000 |
| Polyisobutylene | 15 200 |
| Polydimethylsiloxane | 24 400 |
| Poly (vinyl acetate) | 24 500 |
| Poly (methyl-methacrylate) | 27 500 |
| Poly(α-methyl-styrene) | 28 000 |
| Polystyrene | 31 200 |
In dilute polymer solutions or in polymer melts individual polymer chains are arranged into a “Gaussian coil” assembly. If the polymer is not disturbed by external forces, it will have a unique end-to-end distance.
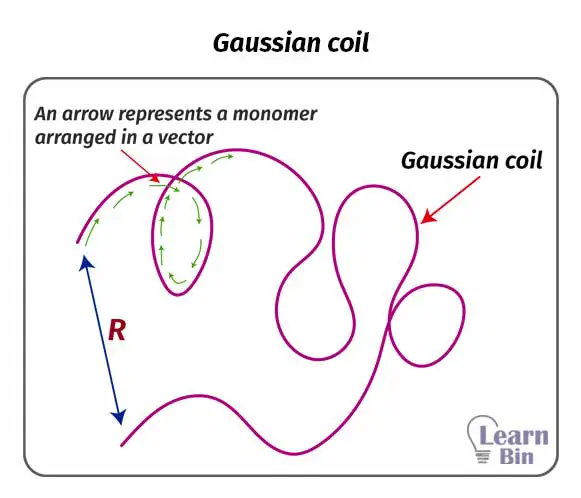
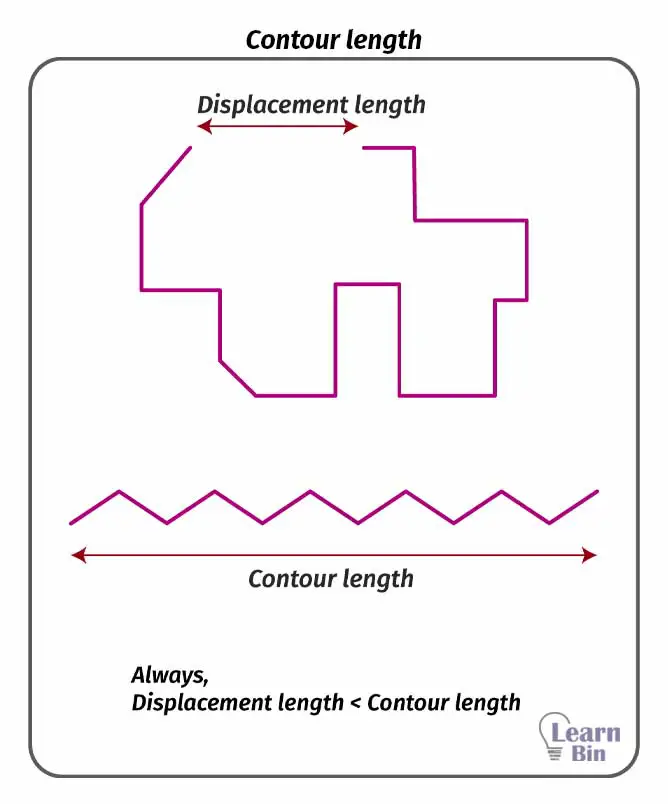
End-to-end distance is the distance between one end and the other end of a linear polymer chain. At a given temperature this value is a specific value for a polymer. The end-to-end distance is always lower than the contour length. Contour length is the maximum length of a polymer when it is fully extended.
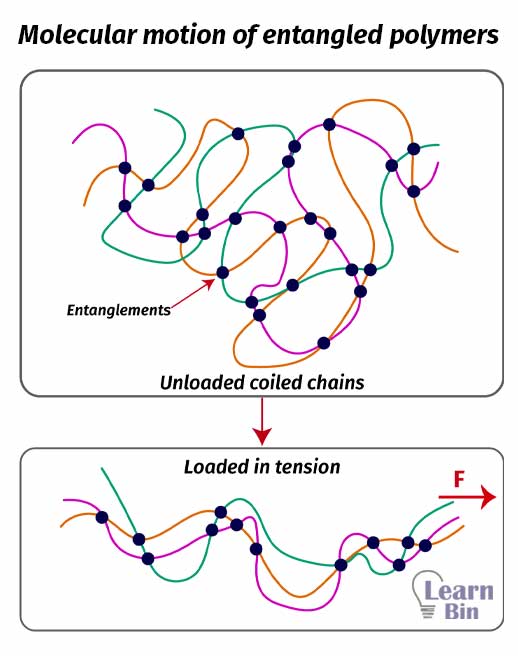
Within the elastic domain, entanglement locations with respect to the coordination of polymer are hardly changing. However, when the polymer has applied a load, the orientation of the polymer chains in between the entanglements will align with the direction of the load.
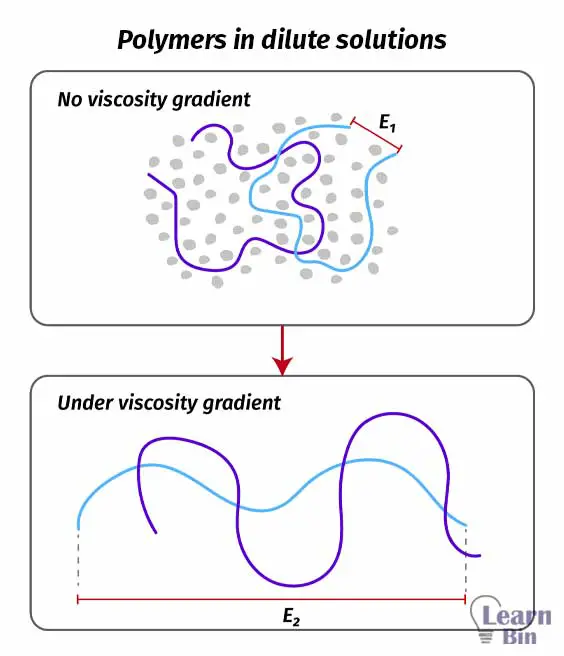
In dilute polymer solutions, polymer-polymer interactions are at a minimum level due to the solvent. Polymer chains have achieved their unique equilibrium and the unique end-to-end distance with the help of the Brownian motion of the individual solvent molecule. Once the solution is applied a force and allowed to flow, solvent molecules will drag the polymer chains with them. Therefore, the polymer chains will also flow along the force. This deformation force is large compared to the Brownian motion. Therefore, under flow conditions effectiveness of the Brownian motion is negligible. Such flow conditions improve the end-to-end distance of the polymer chains.
In concentrated polymer solutions or polymer melts the transmission of deforming load is primarily governed by the interaction between neighboring polymer chains. When we apply a load on the polymer melt, at the beginning entanglement points act as cross-linking points. Therefore, the response to stress is similar to cross-linked rubber. After a time entanglements can slip over and starts to flow.

The cover image was designed using an image by Purpy Pupple, licensed under CC BY-SA 3.0, via Wikimedia Commons