More results...

When explaining the thermal behavior of polymers, at sufficiently low temperatures all polymers are hard and rigid. As temperature rises, each polymer eventually obtains sufficient energy to enable its chains to move. This movement makes the polymer behave like a viscous liquid.
Similar masses of the three different morphologies (amorphous, semi-crystalline, and crystalline) of the same polymer are taken and the variation of its specific volume is observed with respect to the temperature.
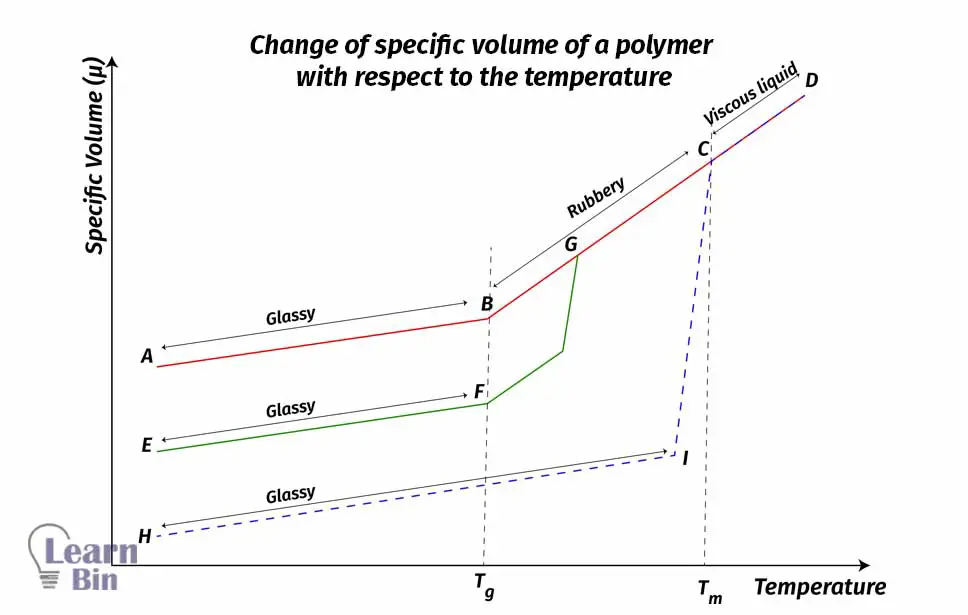
The A-D curve represents the thermal behavior of the amorphous polymer. Initially, it has the highest volume of semi-crystalline and crystalline polymers. From point “A” to point “B” the polymer is in a glassy state. As the temperature increases, intermolecular forces like hydrogen bonds and dipole-dipole bonds are being broken down.
So, the polymer chains become more flexible. They start to slip each other. So, at point “B” the polymer starts to obtain a rubbery texture. The temperature at which the polymer became a rubbery texture is the Glass transition temperature (Tg)
If the temperature continuously increases, the polymer texture becomes a rubbery state of viscous liquid. (B-C curve). At point “C” the polymer has become a completely viscous liquid. At this temperature, polymer molecules are free to move through the polymer matrix. However, amorphous polymers do not show sharp melting points. Their melting temperature is somewhere in between points “B” and “C”. If the temperature is increased beyond the melting temperature, the viscosity of the polymer is reduced.
The perfectly crystalline polymer has the lowest volume compared to the amorphous and semi-crystalline polymers. The H-D curve represents the thermal behavior of a perfectly crystalline polymer. They do not show any glass transition. Crystalline polymers directly become melt states from a glassy state as the temperature increases. At this point, “I” polymer crystals are being melted.
This temperature is the crystalline temperature (Tc). When the temperature increases beyond the crystalline temperature, the polymer becomes a viscous liquid. This particular temperature is its melting temperature. Generally, perfectly crystalline polymers have the highest melting temperatures. Because due to the crystalline structure, they have strong intermolecular forces. So, it needs high energy to break down the intermolecular forces.
Semi-crystalline polymers have intermediate initial volume. They show both glass transition temperatures and sharp melting temperatures. E-A curve shows the thermal behavior of a semi-crystalline polymer. Glass transition temperature of semi-crystalline polymers is higher than the Tg of amorphous polymers. And the Tm is lower than the Tm of crystalline polymer.
When the polymers at below their glass transition temperature the motions of the polymer chains are frozen. When sufficient thermal energy is supplied, chain segments move cooperatively and a transition from a glassy state to a rubbery state begins to take place. This transition is the “Glass transition” of the polymers. Glass transition is a reversible process. Several factors affect the glass transition temperature of polymers.
The most important factor that affects the glass transition temperature is the flexibility of the polymer chains. It is a measure of the ability of polymer chains to rotate. Polymers with flexible chains have low Tg and rigid chains have high Tg. Normally polymer chains with [CH2-CH2], [CH2-O-CH2], and [Si-O-Si] links are very flexible. So, they have low Tg values. Polymers with benzene rings are rigid, so they have high Tg values.
| Polymer | Tg (℃) |
| Poly dimethyl siloxane | -123 |
| Polyethylene | -93 |
| Cis- polybutadiene | -85 |
| Polyphenylene oxide | +85 |
Polymers with bulky pendant groups prevent the rotation of the backbone. Therefore with the increase of the bulkiness of the pendant group, the Tg will also be increased. This is the steric effect.
| Polymer | Pendant group | Tg (℃) |
| polyethylene | -H | -85 |
| Polypropylene | -CH3 | -20 |
| Polyvinyl acetate | -O-CO-CH3 | 28 |
| Polystyrene | Phenyl group | 100 |
The configurational effect such as cis-trans isomerism and tacticity also affect the Tg of polymers. Different stereo structures of the same polymer have different Tg values. For example, isotactic, syndiotactic, and atactic morphologies of polymethyl methacrylate have three different Tg values.
| Morphology | Tg (℃) |
| Isotactic | 105 |
| Atactic | 45 |
| Syndiotactic | 115 |
As well, the cis and trans isomerism of polybutadiene has two different Tg values. (cis-polybutadiene -165K and trans-polybutadiene – 255K)
The Tg is increased with the increase of the molar mass of the polymer. But at very high molar masses Tg is constant.
When crosslinks or branches are present in the polymer, molecular motions are restricted. So, Tg is increased.
Melting temperature (Tm) is the temperature at which the crystalline phase of a polymer becomes a viscous liquid. Several factors affect the melting temperature of polymers. Those are,
The symmetry of the chain affects both Tm and the ability to form crystals. Polymers like polyethylene and Teflon are highly symmetrical. So, their Tm is also high. Chains containing irregular units reduce the ability of a polymer to be crystallizing. Therefore, Tm would be
lower. Polymers with para substitutions have axis symmetry. So, they can crystallize easily. The incorporation of trans double bonds maintains the chain symmetry and can crystallize.
Any interaction between chains in the crystals will help to hold the structure together. This will increase the melting temperature. Polymers containing polar groups such as -Cl, -CN, -OH can have strong dipole-dipole interactions. Polymers with hydrogen bonds such as polyamides have high melting points.
If a polymer chain contains a large number of pendant groups, it will increase rigidity. But it also increases the difficulty of close packing to form crystals. If the pendant groups are arranged in a regular pattern along the chain, it will help to form crystals. Isotactic and syndiotactic polymers have a regular pattern, so, they will tend to be crystallized. Atactic polymers have no regular pattern so, they will be amorphous.
Repeating units and pendent groups have no regular stereochemical arrangement through the polymer backbone.
All the repeating units and pendent groups have the same regular stereochemical configuration.
Repeating units and pendent groups have alternative stereochemical configurations.
If the chains are sufficiently branched, the polymer cannot peak into crystals and Tm will be lower. As an example, linear polyethylene has high density and high Tm than branched polyethylene.
The chain ends of the polymer molecules are always free to move. Let’s consider an equal mass of the same polymer with high molar mass and low molar mass. The polymer with high molar mass has a smaller number of chain ends. So, it has a high Tm. Thus, the polymer with low molar mass has a higher number of chain ends. So, it has a low Tm.
| Polymer | Melting temperature (℃) |
| Polypropylene 2000 g/mol | 33.8 |
| Polypropylene 3000 g/mol | 169.8 |

Cover Image was designed using Image by LillyCantabile from Pixabay