More results...
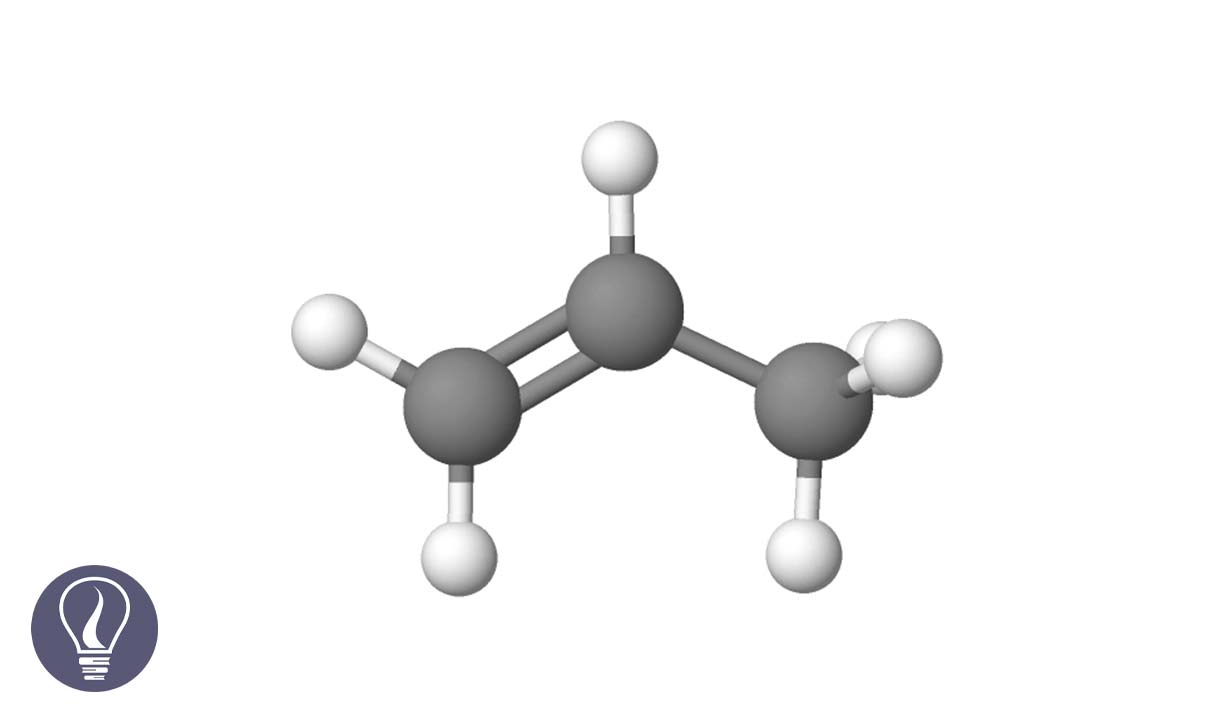

Alkenes are a type of aliphatic hydrocarbons, that contain one or more double bonds in the hydrocarbon chain. The hybridization of the carbon, that has been included in double bonds, is sp2. Since there are double bonds in alkenes, they are also a type of unsaturated hydrocarbons.
So, they could undergo electrophilic addition reactions from the cleavage of the pi bond. Therefore, alkenes are used in the preparation of polymers like polythene, polypropylene, etc.
The empirical formula of alkenes that has only one double bond is CnH2n. Carbon atoms bond linearly to form acyclic alkenes. Sometimes there are branches in the carbon backbone. Cyclic alkenes can also be found when carbon atoms bonded as cycles. In this article, we discuss linear alkenes that only have one double bond.
Alkenes are nonpolar compounds. Therefore, they have only Wan-der Waals interactions between molecules as secondary interaction forces. When the number of carbon atoms increases, the molecule gets bigger, so it gets a higher surface area. Thus, the Wan-der Waals forces also increase. When the number of carbon atoms are increasing it could be a liquid or a solid at room temperature.
Due to the double bond, alkenes could form diastereoisomers as cis and trans structures. The isomerism also affects the melting and boiling point of the alkenes. As an example, butane forms three different isomers 1-butene, trans-2-butene, and cis-2-butene. Although the number of carbon atoms is the same in each structure, they have different melting and boiling points.
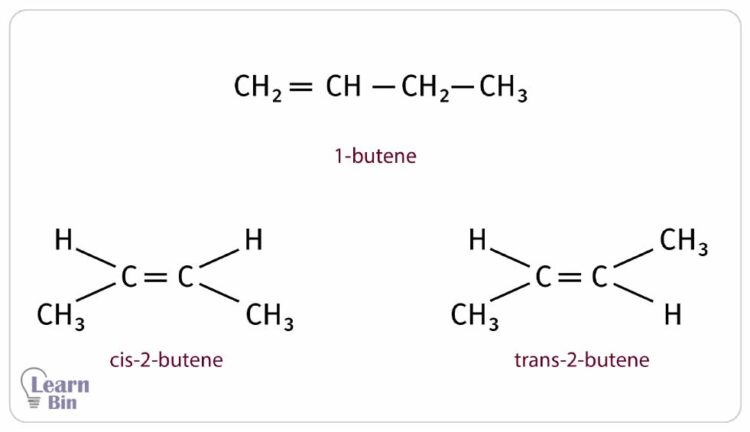
| Isomer | Boiling point (oC) | Melting point (oC) |
| 1-butene | -6.5 | -185 |
| trans-2-butene | 1 | -106 |
| cis-2-butene | 4 | -139 |
Usually, when an alkene has a linear structure it gets a higher surface area. Therefore, the wander Waals forces are high. Thus, the melting points and the boiling points of linear alkenes are high compared to the branched alkenes with a similar number of carbon atoms.
Since alkenes are nonpolar compounds, they do not dissolve in polar solvents like water. But they are soluble in organic solvents.
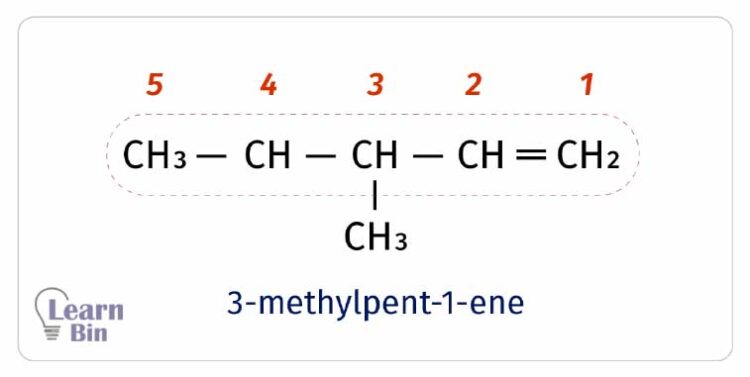
Linear alkenes without any pendent groups and diastereoisomers are named according to their number of carbon atoms in the carbon backbone. The stem name is added “ene” suffix to name an alkane.
| Number of C atoms | Formula | Stem name | Name |
| 2 | C2H4 | Eth | Ethene |
| 3 | C3H6 | Prop | Propene |
| 4 | C4H8 | But | Butene |
| 5 | C5H10 | Pent | Pentene |
| 6 | C6H12 | Hex | Hexene |
| 7 | C7H14 | Hept | Heptene |
| 8 | C8H16 | Oct | Octene |
| 9 | C9H18 | Non | Nonene |
| 10 | C10H20 | Dec | Decene |
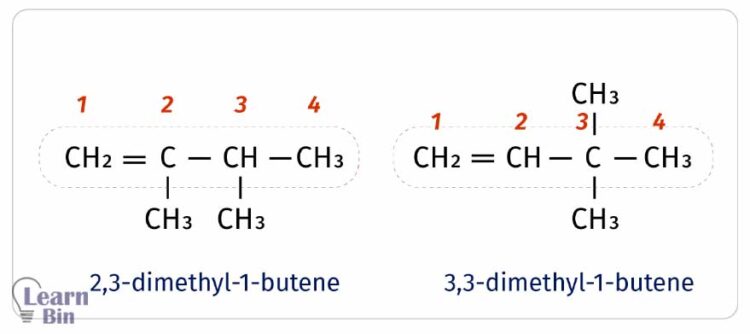
There can be different structural formulas for one empirical formula. As an example, C4H8 empirical formula has two different structural formulas as follows.
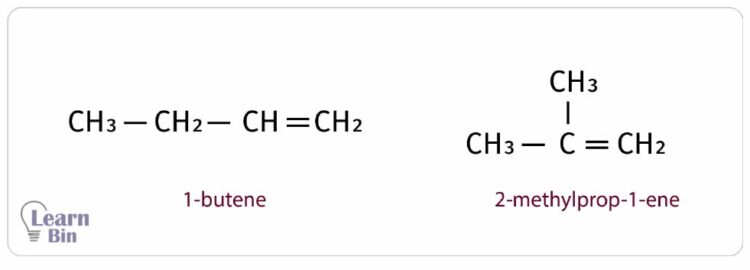
IUPAC nomenclature is introduced considering the structural formula as well as the empirical formula.
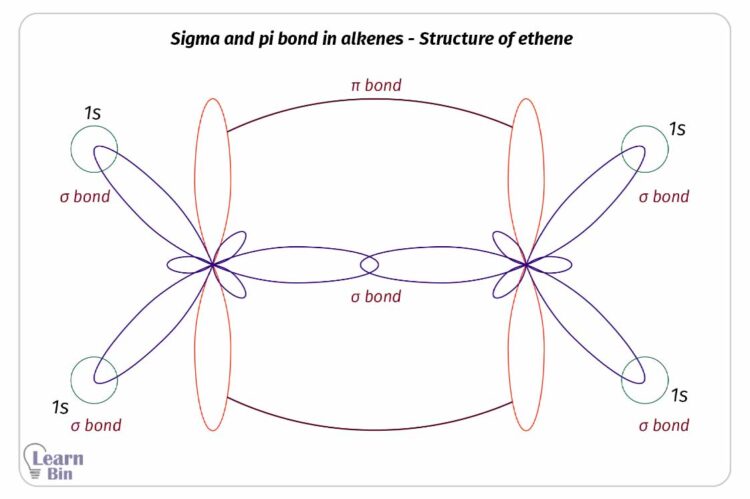
In an alkene, there are two types of bonds which are pi bonds and sigma bonds. Carbon atoms that form double bonds are sp2 hybridized. Carbon atoms that only form sigma bonds are sp3 hybridized.
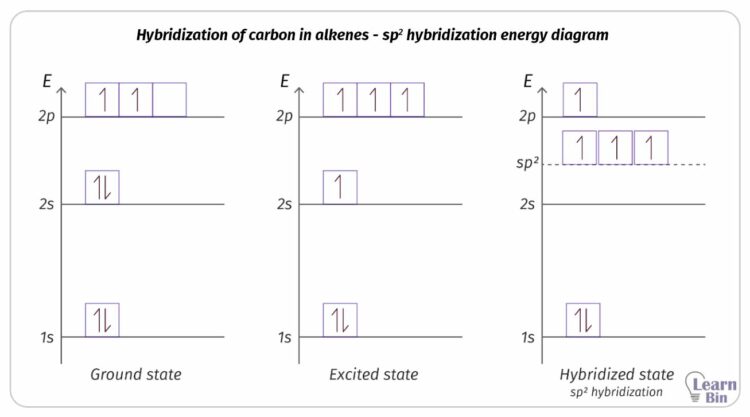
At ground state, carbon has four electrons in its valence shell. The electron configuration of carbon at the ground state is 1s2 2s2 2p2. When it is supplied energy, an electron in 2s orbital moves 2p. Thus, four unpaired electrons are obtained. It can be represented in an energy diagram as follows (figure 05).
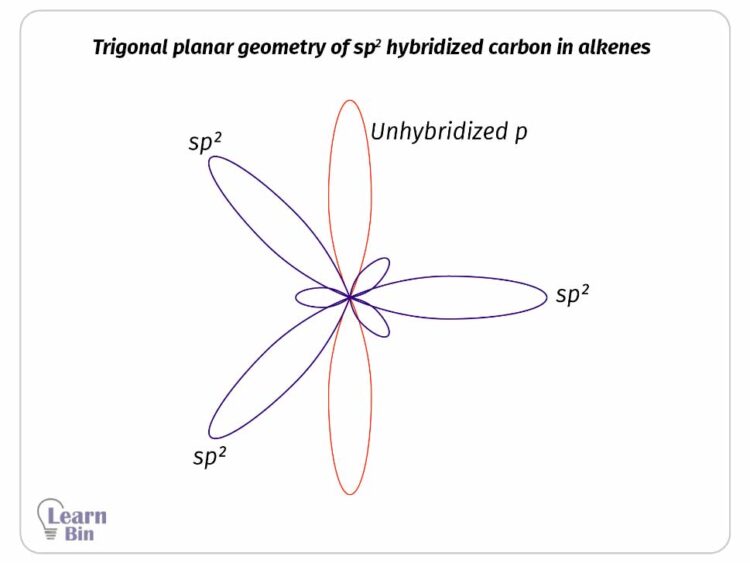
Two of the 2p atomic orbitals and 2s orbital are hybridized and form three sp2 hybridized orbitals. After hybridization, it remains one un-hybridized 2p orbital that contains one lone electron. The three sp2 hybridized orbitals are similar in size, shape, and energy. They have lower energy than the un-hybridized 2p orbital and higher energy than the 2s orbital.
These sp2 hybridized orbitals remain trigonal planar geometry and 120° angle to each other. The un-hybridized 2p orbital remains perpendicular to the trigonal planar. In alkenes C=C double bond is formed by the linear overlapping of sp2 hybridized orbitals. A C-H bond is formed by the linear overlapping of the sp2 hybridized orbital and un-hybridized s orbital of hydrogen.
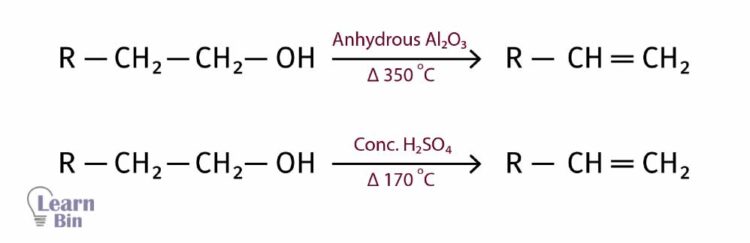
If there is hydrogen on the carbon next to the -OH bonded carbon in an alcohol, such alcohols are treated with anhydrous aluminum oxide and heated up to 350 0C to obtain alkenes.
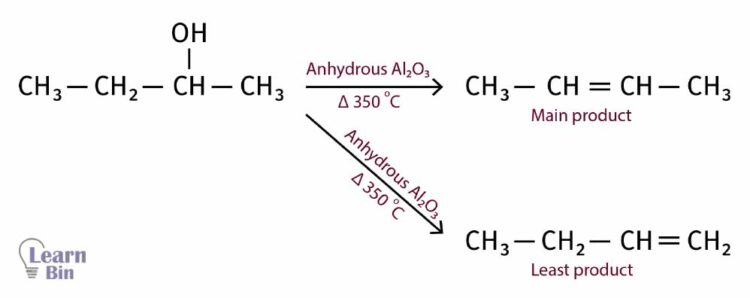
A similar result can be obtained using concentrated sulfuric acid and heating up to 170 oC. Here, the -OH group and the hydrogen in the neighboring carbon will be eliminated as a water molecule. If there are two neighboring carbon atoms that have hydrogen, hydrogen will be eliminated from the carbon with the least hydrogen.
If there is hydrogen on the carbon next to the halogen-bonded carbon in an alkyl halide, such alkyl halides are treated with alcoholic KOH. Here, the halogen and the hydrogen in the neighboring carbon will be eliminated as a hydrogen halide molecule.
If there are two neighboring carbon atoms that have hydrogen, hydrogen will be eliminated from the carbon with the least hydrogen.

The cover image was created using the molecular editor from Molview.org