More results...


When the rate of formation of a component in a system is equal to the rate of consumption of that component, the system is said to be in a steady state. Here, macroscopic properties do not change with time. The properties measured considering the system as a whole, are called macroscopic properties. Let’s consider a few steady systems,
However, the above systems are not equilibrium systems. To be an equilibrium system, there should be,
Let’s consider the following reaction,
A ↔ B
In the beginning, component A is added to a closed system. An amount of mols from component A is converted into product B. the rate of formation of B is maximum in the beginning. The rate is being decreased with time. In the beginning, there is no B in the system. But after B is formed, some mols of B are converted to component A,
Because there is no B in the beginning, the rate of formation of A from B is zero. But that rate increased with time. After a while when the temperature is constant, the rate of "forward reaction" and the rate of reverse reaction will be equal. Both reactions occur at the same rate if there is no external influence. At this point, the system is said to be in ' dynamic equilibrium '.
Rf = Rr
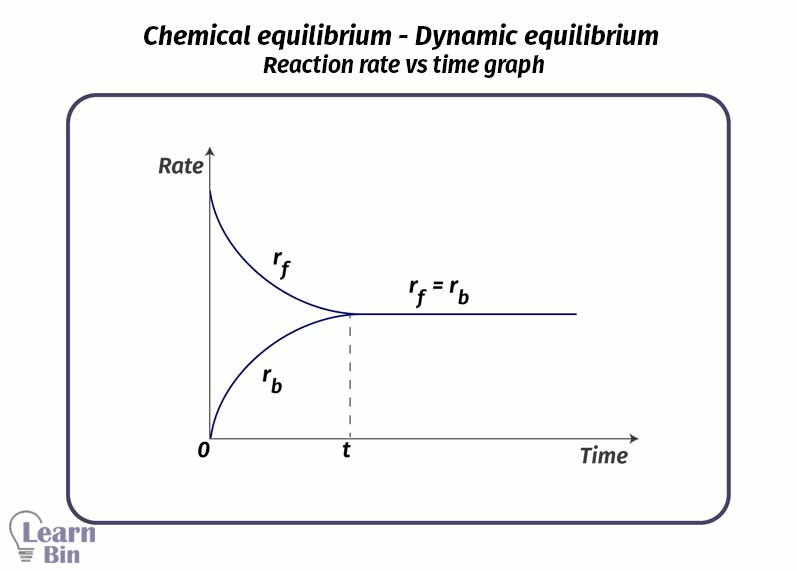
In an equilibrium system, there are both reactants and products. In this system, macroscopic properties such as mols, mol fractions, and concentration remain constant. After became equilibrium there are three types of relationships between the concentrations of products and reactants.
[Reactants] > [Products]
[Reactants] = [Products]
[Reactants] < [Products]
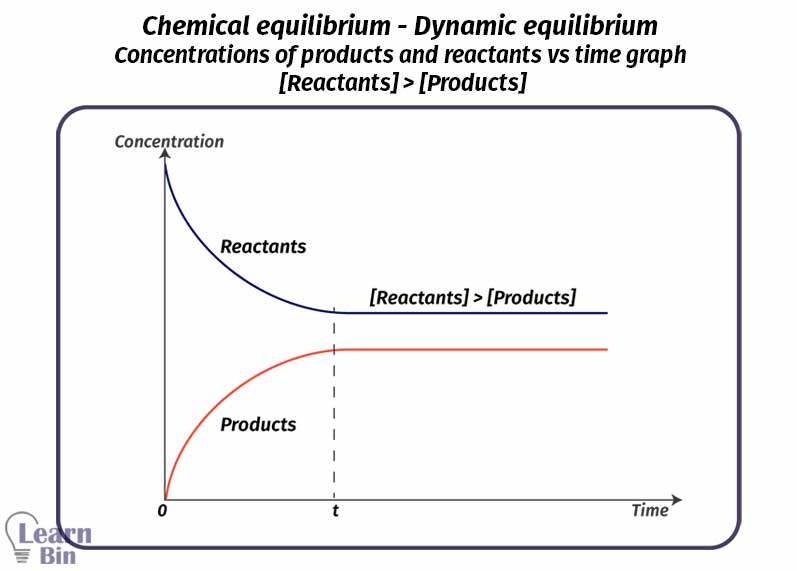
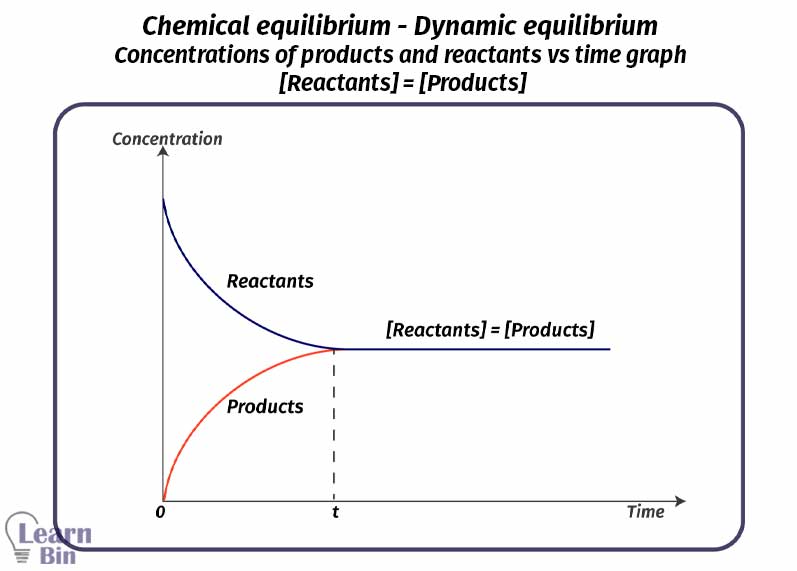
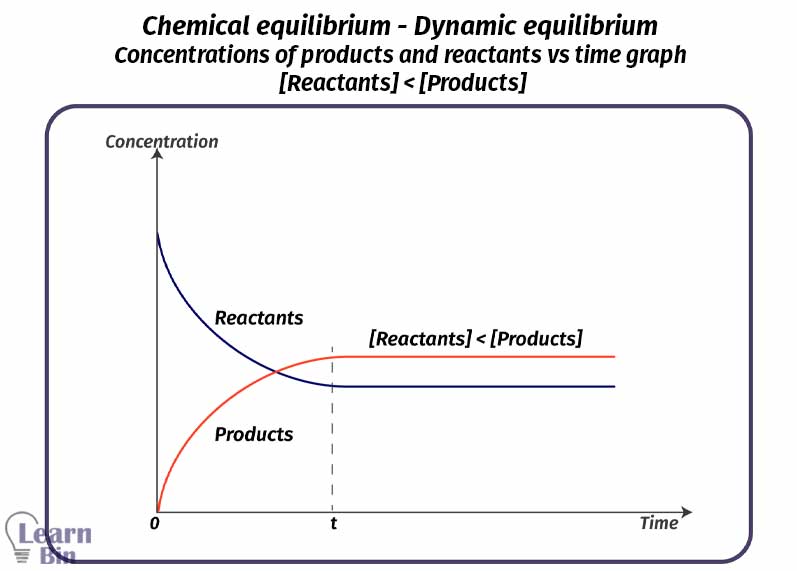
If an external influence occurs in a balanced system in such a way that the equilibrium is broken, the system will return to equilibrium by carrying out more of the forward or backward reaction and equalizing the reaction rates.
Some of the external influences are the concentration of each component, temperature, partial pressure, etc.
The equilibrium point for any reaction is not fixed. It changes when the reaction conditions are changed. In a system in dynamic equilibrium, if any factor that brought about that equilibrium is inhibited, the system will rearrange itself so that the inhibition is minimized.

The cover image was created using an image by Tuhin khamaru from Pixabay