More results...


Density is defined as the mass of a unit volume of any substance. If a substance has “m” mass and “V” volume, the density of that substance can be written as follows.
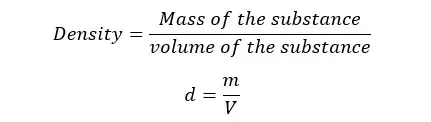
SI unit of density is kg m-3 (kilograms per cubic meter).
Density is constant for each substance. It only depends on the type of the substance. So density is an intensive property.
Examples of density test questions - Question 01
At 4℃, the density of pure water is 1000 kg m-3. Express the density of water in grams per cubic meter (g cm-3)
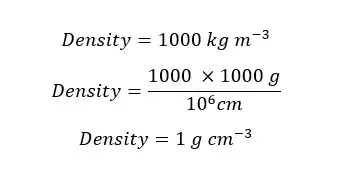
Examples of density test questions - Question 02
Find the mass of 500 cm3 of pure water at 4℃.
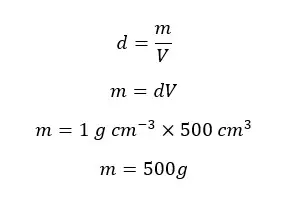
Examples of density test questions - Question 03
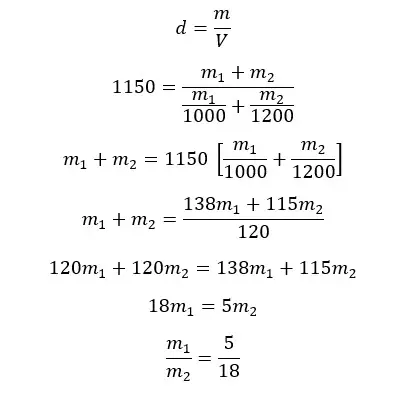
The densities of two liquids A and B are 1000 kg m-3 and 1200 kg m-3 respectively. A homogeneous mixture is obtained by mixing A and B. The final mixture has a density of 1150 kg m-3. By considering there is no volume change when mixing, find the mass and volume ratios between A and B in the mixture.
Mass ratio
Let's take the masses of A and B are m1 and m2 respectively.
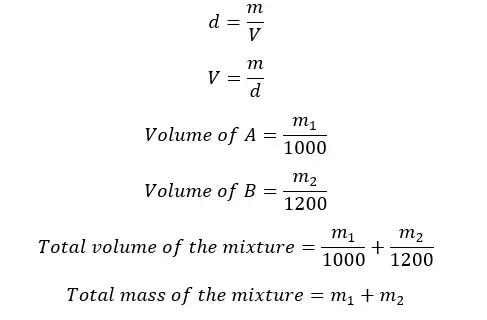
Let's apply the equation for density for the mixture.
Volume ratio
Let's take the volumes of A and B are V1 and V2 respectively.
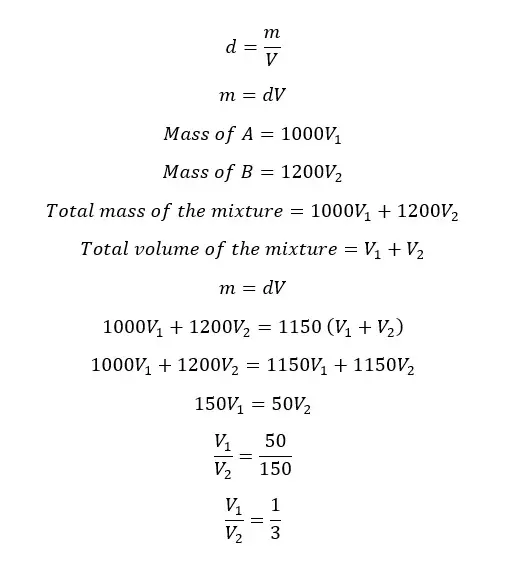
The ratio between the density of any substance, to the density of water is called the “Relative density”. Relative density is a unitless and dimensionless quantity.
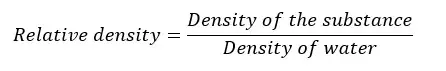
Examples of relative density test questions - Question 01
Find the relative density of coconut oil. (The density of coconut oil is 890 kg m-3 and the density of water is 1000 kg m-3)
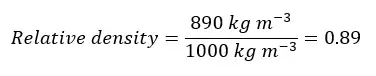
| Material | State of the material | Density (kg m-3) | Relative density (Also equal to density in g cm-3) |
| Water (at 40C) | Liquid | 1000 | 1.000 |
| Water (at 00C) | Liquid | 999.8 | 0.9998 |
| Water (at 200C) | Liquid | 998 | 0.998 |
| Hydrogen (at STP*) | Gas | 8.99 x 10-2 | 8.99 x 10-5 |
| Nitrogen (at STP) | Gas | 1.251 | 0.001251 (1.251 x 10-3) |
| Air (dry air at STP) | Gas | 1.275 | 0.001275 (1.275 x 10-3) |
| Ice (at 00C) | Solid | 917 | 0.917 |
| Sea water | Liquid | 1020 - 1035 | 1.02 - 1.035 |
| Blood (at 370C - average human body temperature) | Liquid | 1050.6 | 1.0506 |
| Diamond | Solid | 3515 | 3.515 |
| Magnesium | Solid (metal) | 1738 | 1.738 |
| Aluminum | Solid (metal) | 2700 | 2.700 |
| Iron | Solid (metal) | 7874 | 7.874 |
| Lead | Solid (metal) | 11340 | 11.34 |
| Mercury | Liquid (metal) | 13534 | 13.534 |
| Gold | Solid (metal) | 19300 | 19.3 |
| Platinum | Solid (metal) | 21450 | 21.45 |
| The core of the Earth | 9820 - 13300 | 9.82 - 13.3 | |
| Neutron star | Solid | 3.7 x 1017 - 5.9 x 1017 | 3.7 x 1014 - 5.9 x 1014 |
| The nucleus of an atom (average) | 2.3 x 1016 | 2.3 x 1013 | |
| Sun (average) | 1408 | 1.408 |
Also to calculate the density of a gas you can use 'The ideal gas equation'.
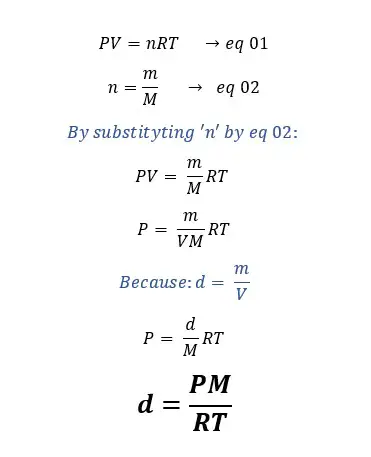
Where,

Table 01: Some data were referred from WolframAlpha.com
The cover image was created using an image by Zaccaria Boschetti from Pixabay
It was really vital because my lecturer always choose question from this site..but since I have now come across this site he is in great trouble.