More results...


Depending on the interactions between water molecules and gas molecules, gases are dissolved in water. Because the natural water resources are in contact with air, gases like oxygen and carbon dioxide are dissolved in water naturally.
When it comes to water quality, dissolved oxygen level (DO) is an important parameter. The dissolved oxygen (DO) levels in natural water bodies and waste waters mainly depend on the physical, chemical, and biochemical activities in the water source. Dissolved oxygen analysis is a fundamental test in water pollution evaluation and waste treatment process control.
There are several methods for determining DO levels in water samples. Some methods are titrimetric methods, and some are in situ testing methods.
Here we are discussing three methods.
The iodometric method also known as the Winkler method is a titrimetric method. The membrane method and Optical probe method are in-situ testing methods.
The iodometric method is the most precise method of analyzing DO. Divalent Manganese solution (MnCl2) and strong alkali solution were added to the sample. In the presence of the base, divalent Manganese ions are oxidized to Mn (iv) resulting in manganese dioxide (MnO2) by Oxygen present in the sample.
The generated MnO2 is reduced to the divalent state (Mn(IV)) by using an iodide solution in an acidic media. liberated iodine (I2) is titrated with thiosulphate solution in the presence of starch as the indicator. The reactions that occurred in the Winkler method can be summarized as follows.
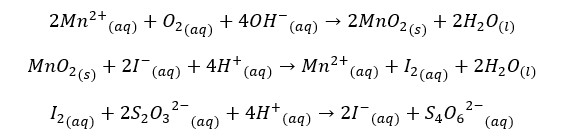
The iodometric method is interfered with by some oxidizing and reducing agents. If there are oxidizing agents, they will oxidize iodide ions and liberate iodine. It is a positive interference. If there are reducing agents, they will reduce iodine into iodide ions and give negative interference. Therefore, some modifications are done to minimize interference.
Azide modification is done for samples interfered with Nitrite (NO2-) ions. The Azide modification is done for samples containing Nitrite ions (higher than 50μg) and Fe (II) ions (less or similar than (<=) 1mg). Generally, this method is used to analyze wastewater, effluent, and stream water samples.
Samples are collected in 300 mL BOD bottles. When collecting samples, ensure no air bubbles are mixed with the sample.
Just after the collection of the sample, 1 mL MnSO4 and 1 mL alkali-iodide-azide reagent are added to the sample. When adding Manganese sulfate solution and alkali-iodide-azide reagent, a brown color precipitate of Manganese dioxide is formed.
After precipitation is completed, the sample is taken for titration. After taking the sample for titration, 1 mL of conc Sulfuric acid (H2SO4) is added to dissolve the precipitate. In the acidic medium, iodide ions are reducing Mn (IV) into Mn(II) liberating Iodine (I2).
When adding reagents to the sample, some volume of the sample is lost. When titrating we take a volume equivalent to 200 mL of the original sample. This sample is titrated with 0.025M Na2S2O3 solution from blue color to a pale straw color in the presence of starch as the indicator.
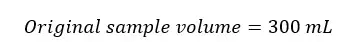
We add 2 mL of reagents (MnSO4 and alkali-iodide-azide reagent) to the above sample. Therefore, some volume of the original sample is lost. The volume of the original sample after adding reagents would be as follows.
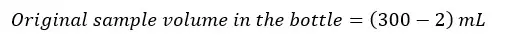
If we should take a volume equivalent to 200 mL of the original sample, the volume of sample to be taken from the reagent added sample would be,
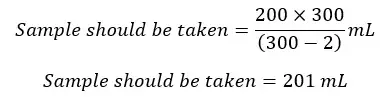
According to stoichiometry,
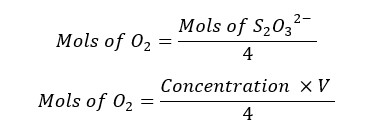
V is the endpoint volume of the titration, and it is taken in milliliters (mL).
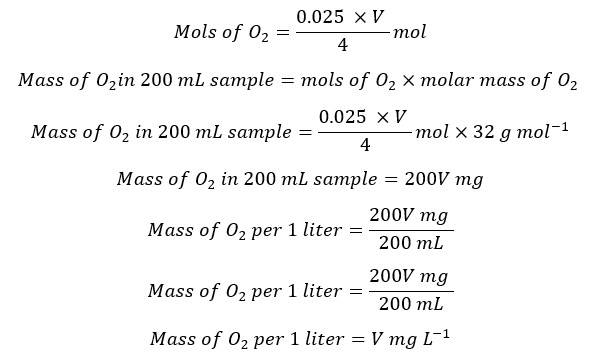
According to the above relationship, the volume of the Na2S2O3 solution is equal to the DO level of the sample.
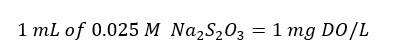
The permanganate modification method is used only on samples that contain a high level of Fe(II) like several hundred milligrams per liter.
Permanganate modification is also included the Azide modification. Here we minimize the interference from both Fe(II) and Nitrite (NO2-) ions.
Samples are collected in 300 mL BOD bottles.
First, 1 mL of KMnO4 solution and 1 mL of KF solution were added to the sample. After adding KMnO4 and KF, 0.7 mL of conc H2SO4 is added by using a 1 mL pipette. MnO4- ions will oxidize Fe(II) into Fe(III).
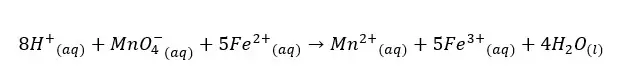
When adding KMnO4 to the sample, the color of MnO4- will immediately disappear because MnO4- ions will be reduced to Mn2+, and Fe2+ will be oxidized to Fe3+. We should add enough KMnO4 to oxidize all the Fe2+ ions in the medium. But make sure not to add a large excess of KMnO4. if the violet tinge of the sample persists for about 5 min, that means all the Fe2+ ions have oxidized.
Then K2C2O4 solution was added to the sample. K2C2O4 will reduce excess MnO4- ions in the medium. Therefore, the solution will be discolored. And the Oxalate ions (C2O42-) ions will form a stable complex with Fe3+ ions.
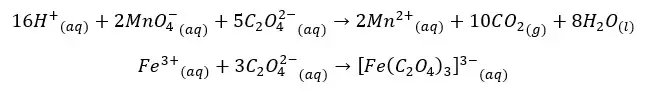
From now, we are continuing the azide modification procedure. But the difference is, here we add 1 mL of MnSO4 instead of 2 mL and 3 mL of iodide azide solution instead of 2 mL.
For the titration, we take a similar volume to 200 mL of the original sample. We have added 1 mL of KMnO4, 1 mL of KF, 0.7 mL of conc H2SO4, 1mL of K2C2O4, 1mL of MnSO4, and 3 mL of alkali iodide azide solution. The total volume of the reagents is 7.7 mL. Therefore, the sample should be taken from the test solution would be,
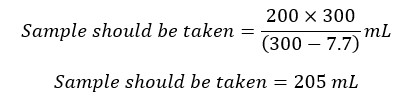
As same in azide modification, the volume of the 0.025M Na2S2O3 solution is equal to the DO level of the sample.
Alum flocculation modification is applied for samples with highly suspended solids. Suspended solids will consume a considerable amount of iodine. We remove suspended solids by alum flocculation.
The sample is collected into a 500 mL to 1000 mL glass bottle without any air bubbles mixed with the sample.
Then 10 mL of a prepared alum solution and 1-2 mL of conc Ammonium hydroxide solution were added to the sample.
Let the solution about 10 min for the sedimentation of the suspended solids.
After 10 minutes the clear supernatant is transferred to a 300 mL BOD bottle until it overflows. Make sure to avoid aeration when the sample is transferred. Then the azide modification method is taken place for the sample.
This modification method is used for samples with biological flocks like activated sludge mixtures.
Add 10 mL of the Copper sulfate-sulfamic acid inhibitor solution to a 1000 mL glass stoppered bottle.
A tube is inserted near the bottom of the above bottle. The test sample is collected in the bottle through that tube. Transfer the sample until it overflows about 25% - 50% of the bottle volume.
Let this solution suspend the solid.
After the suspension, the supernatant is collected in a 300 mL BOD bottle, and azide modification or a suitable modification method is taken place.
Although the Iodometric method or the Winkler method is the most precise method for finding dissolved oxygen level, it cannot be applied to various industrial and domestic wastewater samples. And since it is a titrimetric method, we cannot determine the DO in situ.
The membrane electrode method is a polarographic method where an electrode that has been covered with a membrane is used. The electrode is covered with a membrane because the impurities in the wastewater can cause electrode poisoning or other interferences. Therefore, an oxygen-permeable polymer membrane is used. The membrane is a barrier to impurities.
The electrode is placed in the sample and stirred in the test solution (water sample) to determine the amount of dissolved oxygen. The apparatus will automatically calculate the DO level in the water.
The optical probe method is a luminescence base oxygen sensing method to determine DO in water.
Here, an Oxygen-sensitive optical probe with an appropriate meter and a stirring device is used.
To measure the DO the probe is placed in the sample and started stirring. The apparatus will automatically calculate the DO level. This method is also an in-situ method.

Lipps, W.C., Braun-Howland, E.B. and Baxter, T.E. (2022) Standard methods for the examination of water and wastewater. Atlanta: APHA Press.
The cover image was designed using an image by Michal Jarmoluk from Pixabay