More results...

When considering thermodynamics, is one of the most important parts of science. Thermodynamics describes the flow of heat energy. Principles of thermodynamics are universal; hence they can be applied to describe any kind of systems such as biological system (human body), chemical reactions, mechanical systems (engines), planetary systems, and stars. The universe is the largest thermodynamic system.
Thermodynamics started when the universe started and it will end when the universe ends and the total energy vanishes to nothingness, then thermodynamics will end. However, the theories and laws of thermodynamics began to develop in the early 1800s.
The development of thermodynamics happened before the discovery of atoms and molecules, during the period of the Industrial Revolution (the 1800s). So, the development of thermodynamics is completely empirical, and the principles of thermodynamics were developed based on experimental results and observations. So, this is known as classical thermodynamics.
When a chemical reaction occurs, bonds will break, new ones will be formed, or both will happen. In this process, energy is required to form new bonds, and energy is released when bonds break. So, energy should be transferred between the environment and the reaction system. This energy transfer happens through heat energy.
When principles of thermodynamics are used to describe heat transfers of chemical reactions, that is called chemical thermodynamics.
The discovery of atoms and molecules, and the development of quantum mechanics, help to rationalize thermodynamics. That is called Statistical thermodynamics. Even though it didn’t prove the thermodynamic principles, that helps to improve the validity of concepts.
Every thermodynamic process is based on some form of energy such as potential energy, kinetic energy, and heat energy. When energy is used to do work, it transforms from one form to another along the process. For example, when you drop a rock from some height, the potential energy of the rock will be converted into kinetic energy and heat (thermal) energy along the way.
This section of the article is just an introduction to the concepts of work and energy. They are necessary to understand the theories of thermodynamics. With the development of quantum mechanics and classical mechanics, there are much deeper and more complex concepts to describe work and energy.
Work is defined as the action done on an object that causes a displacement of that object.
For example think about a box sitting on the floor. If you push that box (apply a force on the object) the box will start to move (slide on the floor) and it will be displaced from its original location. That action can be explained as you have done work on that box.
To calculate work below equation is used.
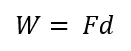
To do work, displacement of the object that force has been applied must occur. If there is no displacement/ movement (d=0) of the object that force has been applied, the work done on that system is zero.
Work can be positive or negative. If the force is applied to the direction of the displacement of the object, it is considered positive work. If the force is applied opposite direction of the movement of the object that is negative work.
Energy is defined as the capacity to do work.
The connection between work and energy is described in the work-energy theorem. If someone does work on an object (system), the energy of motion (kinetic energy) of the object will be changed. So, if an object is in motion, that object can do work on other objects.
Energy is a property of a system or object. Energy can be transferred from one object to another in form of work. There are many forms of energy such as kinetic energy, potential energy, chemical energy, thermal energy, nuclear energy, etc.
But when considering different forms of energy, they all can be placed under kinetic energy and potential energy.
| Forms of energy | |
| Kinetic energy | Vibrational kinetic energy Rotational kinetic energy Light energy Sound energy Heat energy Electrical energy |
| Potential energy | Gravitational potential energy Elastic energy Chemical energy Nuclear energy |
Kinetic energy is the energy of motion. So basically, anything that moves (objects, molecules, atoms, subatomic particles) that has mass, and speed has kinetic energy.
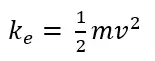
This equation of calculating kinetic energy in classical mechanics only can apply to objects that travel at much less speed than the speed of light (v<<<c).
According to this equation, only moving things that have mass have kinetic energy. But what about photons (radiations)? They are always moving at the speed of light. But they have no mass. So according to classical mechanics (Newtonian mechanics), they shouldn’t contain any energy. But photons are pure energy, and they are in motion. With the development of quantum mechanics, it was discovered that the energy of photons can be categorized as kinetic energy. It can be calculated using the Planck-Einstein relation.
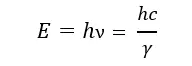
Potential energy is the energy due to position. This can be considered as the stored energy of an object.
Gravitational potential energy can be calculated using the below equation.
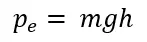
Other than gravitational potential energy, there are many forms of potential energy such as elastic energy.
These are the definitions of some important terms in sense of thermodynamics.
In thermodynamics, a system can be defined as a part of the universe that is interested in an observer. For example, it can be a body of an animal, tree, or beaker that has some reaction happening in it, a machine, or a vehicle.
An open system is a system that which both matter and energy can be transferred between the system and the surrounding.
Ex: Open beaker, Human body, steam engine
In a closed system, only energy can be transferred between the system and the surrounding. No matter can be exchanged.
Ex: Closed beaker
In an isolated system, energy and matter exchange cannot occur between the system and the surrounding. An isolated system is thermally, electrically, and mechanically isolated from the surrounding.
The universe is the only true isolated system that exists. But thermos flask can be considered as a good example.
A homogeneous system is a system whose properties are uniform throughout the system (Ex: a mixture of NaCl and water, a balloon filled with air).
A heterogeneous system is a system whose properties are not uniform throughout the system or/and the system contains more than one phase. (Ex: human body, colloidal solution, a soup, mixture of coconut oil and water)
Surrounding can be defined as the outside of the system and this is the rest of the universe excluding the system that is observed by the observer. Surrounding is where observers stand and make the observations (getting measurements).
The boundary is the border that the system and the surrounding separate. When defining the boundary of a system it has to be very precise.
Depending on the heat transfer through boundaries, there are two types of boundaries.
The universe can be defined as the collection of the system and surrounding.
Ex: Mass (m), Volume (V), Amount of substance (moles – n), Internal energy (U), Entropy (S), Enthalpy (H), Heat capacity
Ex: Temperature (T), Pressure (P), Density, Specific heat capacity
Ex: Pressure (P), Volume (V), Internal energy (U), Temperature (T), Entropy (S)
Ex: Work, Heat
The laws of thermodynamics are the basis of thermodynamics. These laws explain the flow of energy. Laws of thermodynamics explain how energy flows, which direction it flows, and how energy converts to different forms in a thermodynamic system.
What explained in those laws are seems like common sense. But they provide a mathematical foundation to explain thermodynamics. Each law lays an experimental foundation for the introduction of important thermodynamic properties.
There are four fundamental laws of thermodynamics. Those laws are universal. Using these laws energy flow of any type of system in the universe can be described.
This article is just an introduction to thermodynamics. So, each law of thermodynamics will be fully described in separate articles.
Zeroth law of thermodynamics is known as the commonsense law. Even though its name says the zeroth law, this law was established in the early twentieth century, and this was established after the establishment of the first and second laws of thermodynamics. Because this law is much more fundamental to thermodynamics, this law was assigned as the zeroth law.
Zeroth law of thermodynamics is much more fundamental to thermodynamics because it provided the experimental foundation for the introduction of one of the most important thermodynamic properties: Temperature. This law is the basic principle behind the development of the thermometer. This law defines thermal equilibrium. So, let’s find out what the zeroth law of thermodynamics is.
Let’s think about three closed systems: systems A, B, and C. If system A is in thermal equilibrium with system B, and System B is in thermal equilibrium with System C, then system A is also in thermal equilibrium with system C.
Simply the first law of thermodynamics is about energy conversion. This law states that energy cannot be created or destroyed. The only thing possible is to convert one form of energy into another form of energy. This law introduced another important thermodynamic property: Internal energy.
The second law of thermodynamics introduces entropy and this is an important thermodynamic property. This law states that the entropy of a closed system is always increasing.
The third law of thermodynamics introduces another important concept in thermodynamics: Temperature of Absolute Zero. The third law states that at the temperature of absolute zero, the entropy of any crystalline substance is equal to zero.

Atkins, P. Four laws that drive the universe; Oxford University Press: Oxford, 2007.
The cover image was created using an image by Mudassar Iqbal from Pixabay