More results...


Polymer degradation is a process that changes the properties of a polymer. When polymers degrade, their properties are decreased. Degradation is an irreversible process. Polymer degradation is initiated by,
When a polymer degrades it can change its molecular weight, mechanical properties, physical appearance, transparency, brittleness, hardness, color, etc. So, preventing or delaying degradation is important in the economic aspect.
Polymeric molecules are very large on the molecular scale. Their unique properties are mainly a result of their size. Any loss in chain length lowers the mechanical and physical properties.
Polymer degradation can happen in three ways.
This is due to the breakdown of primary bonds.
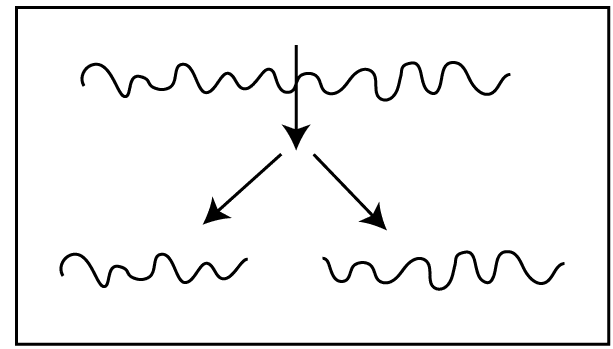
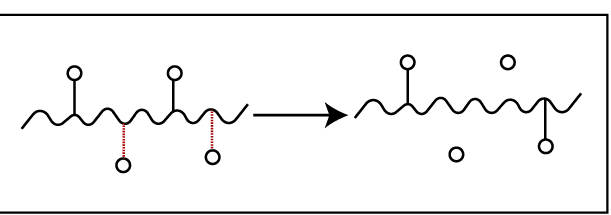
This is due to the breakdown of secondary bonds. Here pendent groups are disintegrated from the polymer backbone. After the breakdown of pendent groups, crosslinks are formed between chains. So, the molecular weight of the polymer, tensile strength, and brittleness are increased. Sometimes, they do not show crosslinking.
E.g.: PVC – PVC removes HCl molecules when degrade.
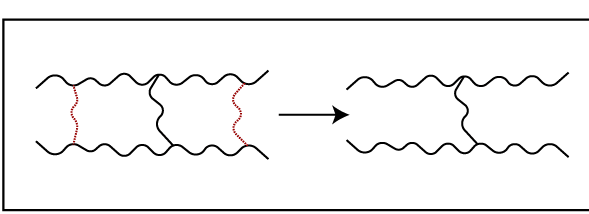
This is due to the breakdown of cross-links.
There are internal and external factors on which polymer degradation is dependent. The stability of a polymer depends on.
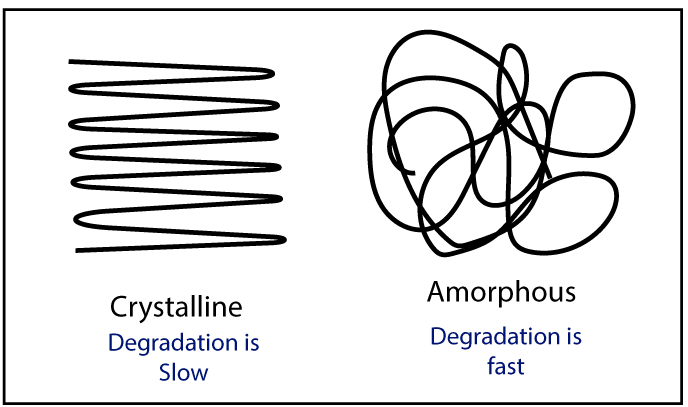
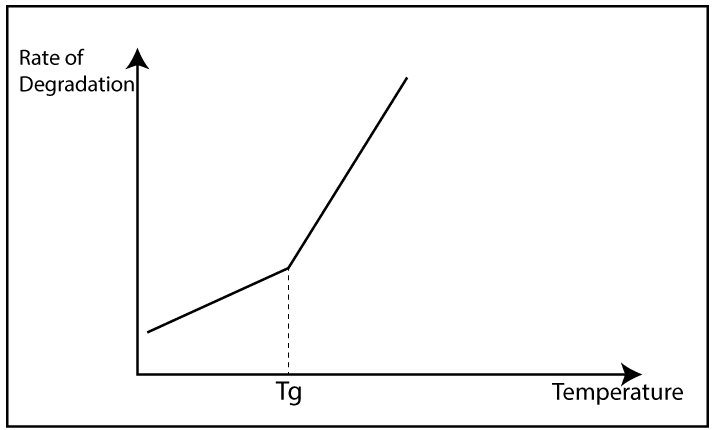
There are mainly two morphologies in the polymer structure. Those are crystalline and amorphous. The crystalline structure slows down the degradation because in crystalline structure polymer molecules are packed well. So, the external O2 can not enter the polymer.
But in an amorphous structure, polymer molecules are randomly arranged. So, external O2 can easily enter the polymer structure. When O2 is present, they react with polymer chains and degradation will happen.
The rate of degradation can be determined by the surface/volume ratio. Higher the O2 concentration on the surface higher its reaction rate. Degradation most of the time is limited to the surface layer.
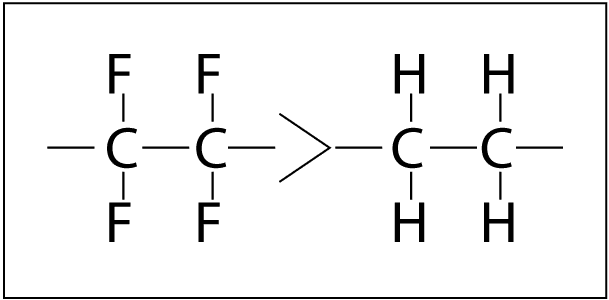
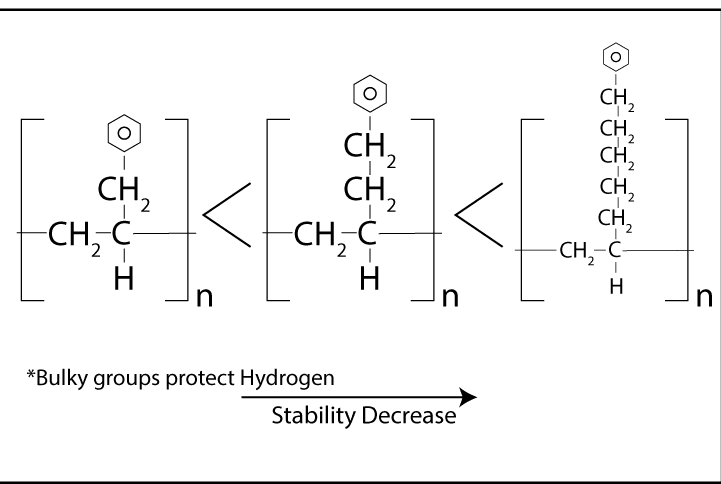
The rate of polymer degradation depends on the strength of chemical bonds in the structure.
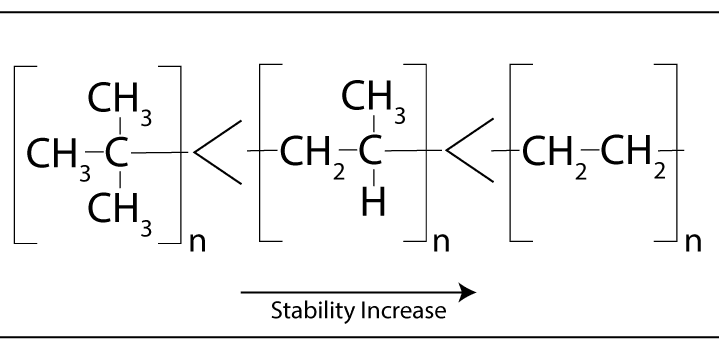
Trace impurities such as initiators, and catalysts that are in the material initiate the degradation process.
Some environmental conditions such as salt, moisture content, and sunlight can initiate degradation. Some sort of energy sources such as heat, radiation, or mechanical stress, and some sort of chemical reagents such as O2, H2, and O3 are needed to initiate degradation. Degradation is categorized according to its energy source and chemical reagent which initiates the degradation process.
Organic and inorganic liquids and salts initiate the degradation process.
Biological agents such as bacteria, and fungi can initiate the degradation process.
When applying mechanical stress, decreases the stability of the polymer.
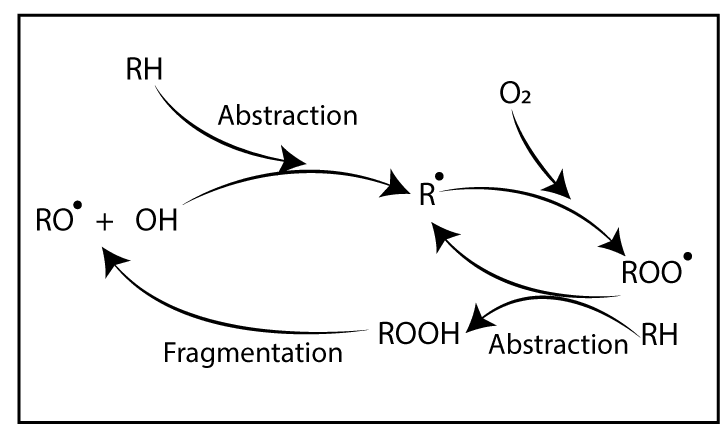
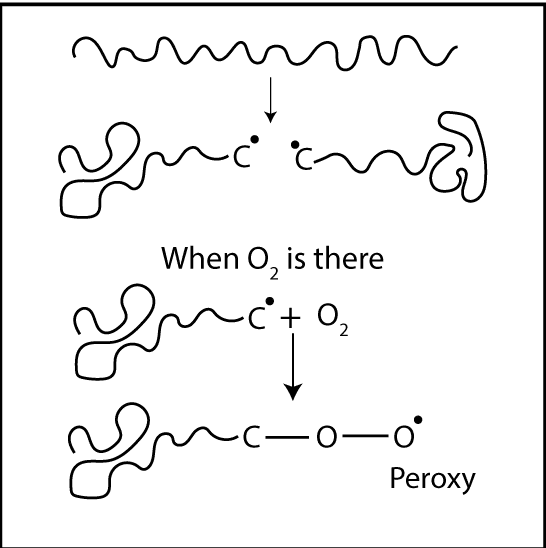
Degradation is a chain reaction. Once a radical is formed, a chain reaction can occur. Free radicals are formed by an energy source. If oxygen is there, it reacts with free radicals. Then, “Peroxy bonds” which have low bond strength are formed. Due to this low bond strength, it can be easily broken down. In other words, degradation has happened.
Mainly degradation happens in two ways.
Degradation occurs from one end of the polymer. Monomer molecules are eliminated from the backbone, one by one.
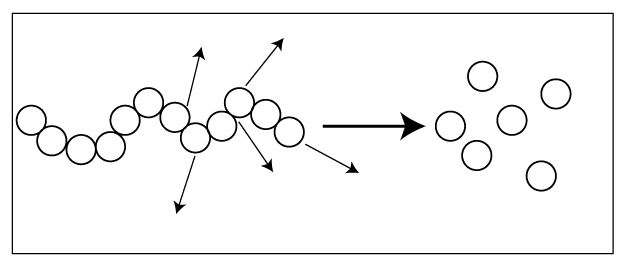
The polymer backbone is randomly broken down.
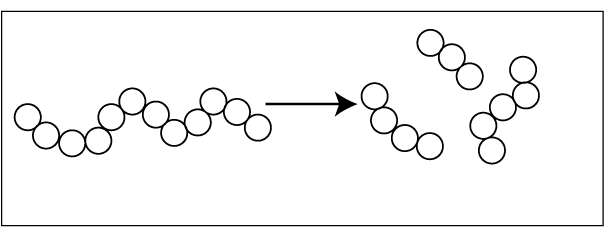
After polymer degradation molecular weight is reduced. The type and amount of degradation depend on several factors,
Polymer degradation is always faster in the presence of Oxygen primarily due to the auto-acceleratory nature of the reaction between Oxygen and carbon-centered radicals. Interaction with Oxygen increases the concentration of polymer alkyl radicals (R*) which creates the highest level of fusion and cross-linking.
Additionally, fragmentation reactions create oxygen-centered radicals (RO*) giving new species. These are “Oxygenated products” that would not be found in the polymer process under air-free conditions.
There are three main steps in the degradation mechanism.
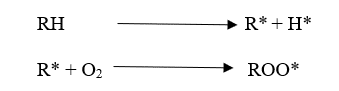
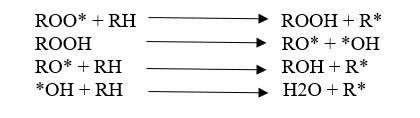

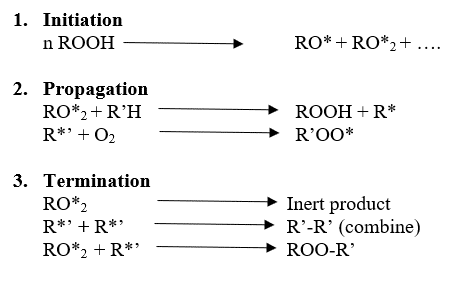
Read more about how to Increase the stability of polymers against degradation here.

Cover photo credits – Photo by Catherine Sheila from Pexels