More results...


Generally, pigments are aggregated dry particles. The aggregation of pigments in paint reduces the scattering of the light compared to the separated pigments. It causes to reduce the hiding power of the paint. Also, large aggregates may result in less uniform films as well. To prepare a paint pigment dispersion, pigments should be perfectly dispersed in a solvent.
The dispersion mechanism of a paint pigment consists of three process steps.
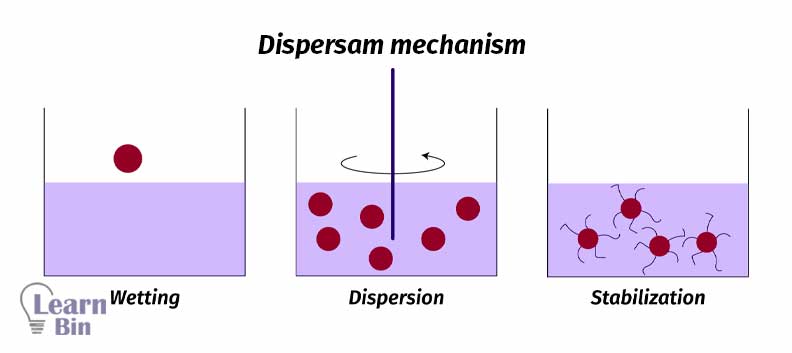
Wetting is the process that is replacing the air present at the interface of the pigment particles with a vehicle (solvent or a binder).
The wetting depends on certain factors like the surface tension of both pigments and solvent, the viscosity of the resin solution, the geometry of the pigments, etc. For good wetting ability, the surface tension should be low.
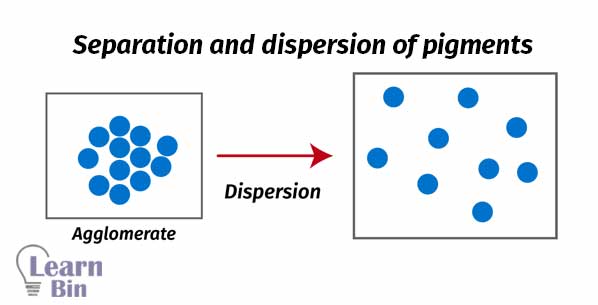
Pigment agglomerates are broken down to primary particles by mechanical energy by using “High-speed stirrers” or “Ball mills”. Due to the stirring pigment particles perfectly spread through the solvent.
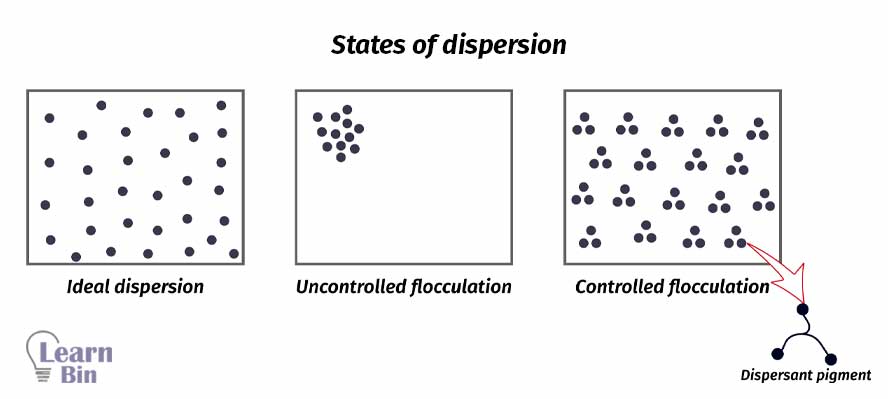
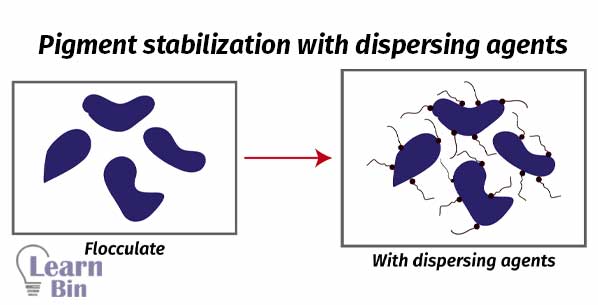
A pigment dispersion can be either completely dispersed or flocculated. If pigment particles are evenly dispersed in the solvent, it is known as an ideal dispersion. In an ideal dispersion of pigment, the hiding power is high. And the paint has a higher gloss. Viscosity is low.
If pigments are not properly dispersed in the solvent, pigment particles remain aggregated. Therefore, the solution has a high viscosity and low hiding power. This is uncontrolled flocculation.
Aggregated particles can be dispersed in the medium using dispersants. Pigments are dispersed in a controlled way. If the pigment affinity groups are not confined to a small region of the dispersion but are distributed in a special fashion over the entire molecule. That dispersant can simultaneously contact more than one pigment molecule giving controlled flocculation.
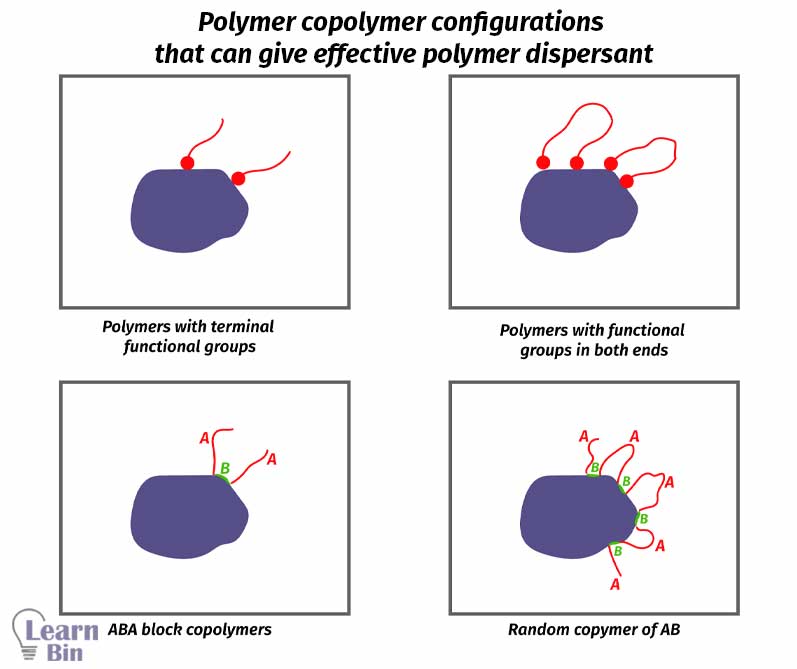
If pigments are not dispersed in the solvent naturally, dispersants are used. Dispersants are substances that are used to disperse substances in a media. There are two types of pigment dispersants which are polymeric dispersants and surfactants.
These are polymers with specific anchoring groups to have strong adsorption to the pigment surface. Polymer chain gives steric repulsion. So, pigment particles will not reaggregate. But if the polymer chains are not sufficiently solvated, polymer chains will collapse onto the pigment surface resulting in pigment aggregation.
The molecular mass of the polymer is also important. If the polymer chain is too short, it will not provide a sufficient steric repulsion and pigments will reaggregate. If the polymer chain is too long, it will fold back and not give sufficient steric repulsion. Therefore, it should have an optimum length.
The pigment surface is interacted with by a functional group or a part of the dispersant. This interaction is called anchoring.
Surfactants are molecules that have both hydrophobic and hydrophilic parts in the same molecule.
| Type of surfactant | Examples |
| Cationic | Ammonium salts |
| Anionic | Phosphates Sulfates |
| Amphoteric | Betaines |
| Non-ionic | Ethoxylates |
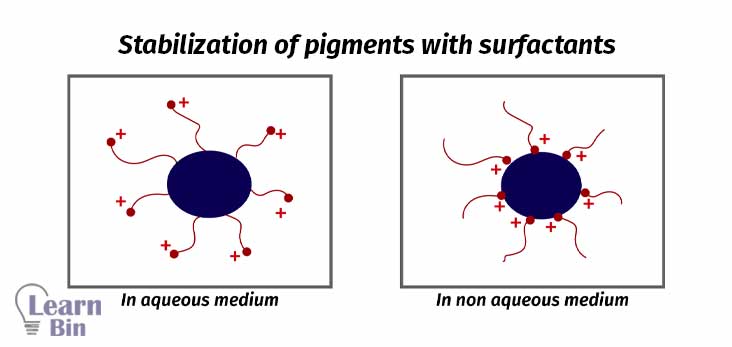
Mostly pigment dispersion is not stable. Pigment particles will reaggregate after mechanical energy is stopped. So, the dispersion should be stabilized.
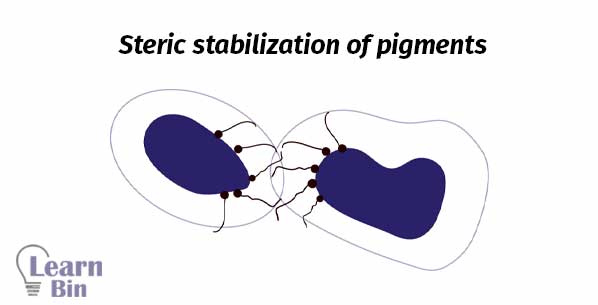
Stabilization is achieved through the adsorption of stabilizing molecules on the pigment surface. So, repulsive forces among the adsorbed species will prevent the reaggregation. There are two stabilization mechanisms.
In liquid dispersion media, ionic groups can adsorb to the surface of colloidal particles forming a charged layer. Counter ions with opposite charges will surround the particles and generate an ' electrical double layer '. This results in coulomb repulsion. Electrostatic stabilization is a kinetic stabilization method
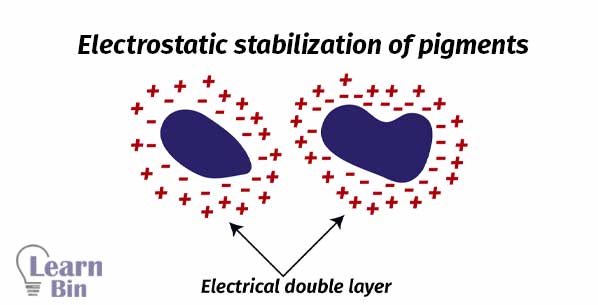
Anionic or cationic polyelectrolytes (polymers with multiple electric charges) are used for electrostatic stabilization.
Steric stabilization is obtained by attaching nonionic surfactants or polymers to the surface. This is a thermodynamic stabilization system.

Thank you for sharing your knowladge.