More results...

Alkynes have one or more triple bonds. A triple bond consists of two pi bonds and one sigma bond. The unsaturation of alkynes is higher than that of alkenes. Alkynes can undergo reactions through the cleavage of their pi bonds. These two pi bonds react with electrophiles independently.
Also, it follows Markovnikov’s rule. The electron density around the triple bond is high. Therefore, electrophiles can be attracted by the triple bond. Thus, alkynes undergo electrophilic substitution reactions.
When compared to alkenes, the reactivity of alkynes is lower than that of alkenes. Because alkynes have triple bonds. And the triple bonds have much higher bond energy. Therefore, it needs higher energy for the cleavage of bonds.
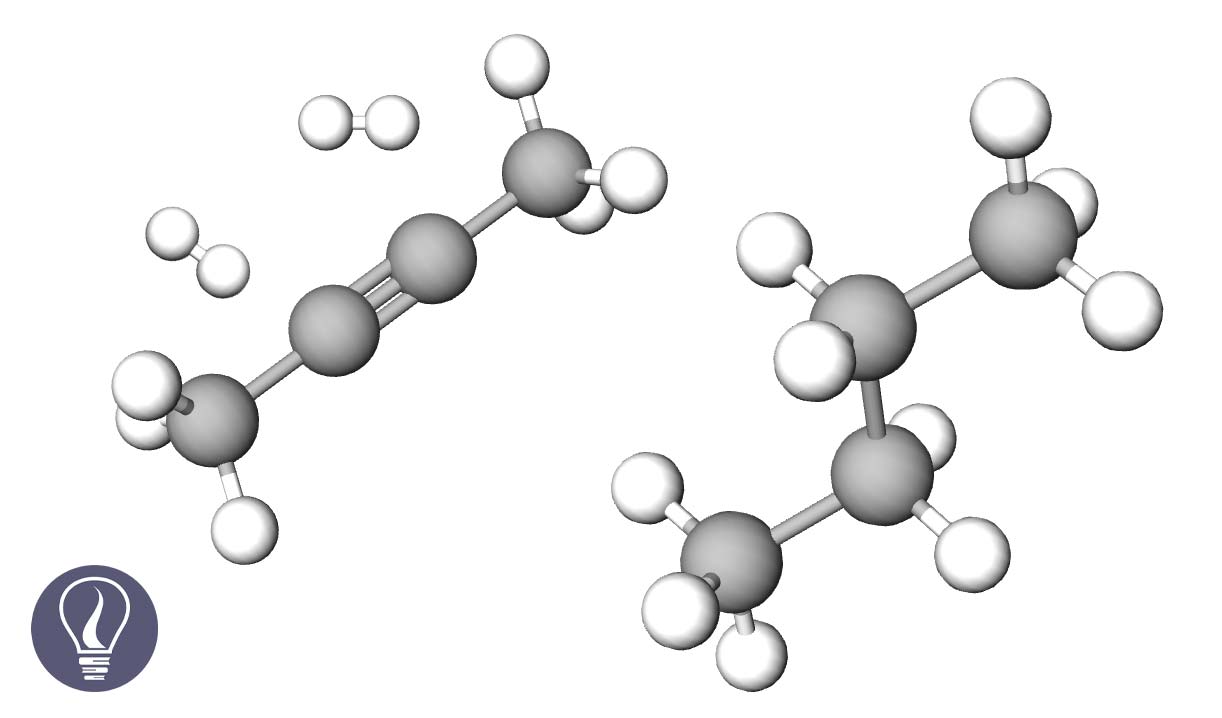
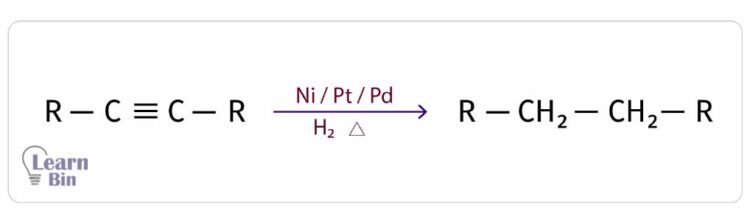
In the presence of Ni (nickel), Pt (platinum), or Pd (palladium) catalysts, alkyne is heated with an H2 air current to get alkane. Here, two pi bonds are broken down, and Hydrogen is added. For 1 mol of triple bonds, 2 mol of H2 are added. The hybridization of carbon changes from sp to sp3.
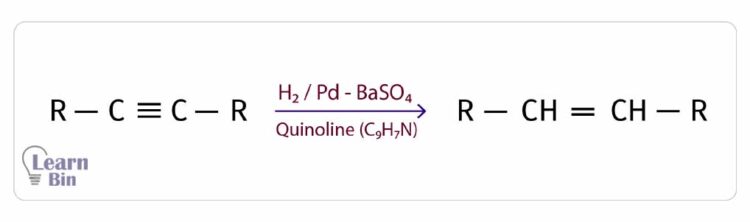
In this reaction, first, the alkyne is reduced into an alkene and further reduced into an alkane. If this reaction should be stopped at the alkene stage, it uses a Pd catalyst deposited on BaSO4 and deactivated by quinoline. It reduces the catalytic activity, and the reaction is stopped at the alkene stage.
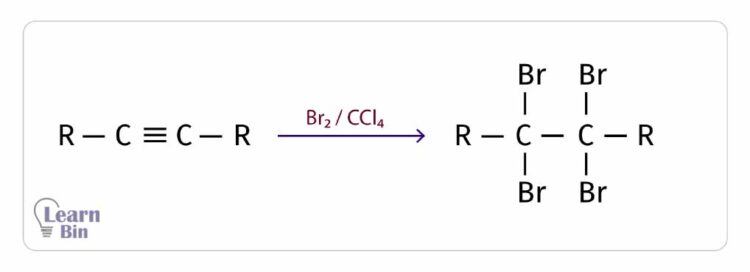
Bromine is added to the alkyne in a medium of CCl4. Water is not used as a reaction medium here. Because, if water is used, the -OH group is also added to the alkyne. Also, CCl4 is a non-polar solvent. Therefore, Br2 can be easily dissolved in CCl4 since Br2 is also a non-polar molecule.
In this reaction, it can be observed that the red color of the Br2 liquid turns colorless. Therefore, this reaction can be used to identify unsaturation in organic compounds.
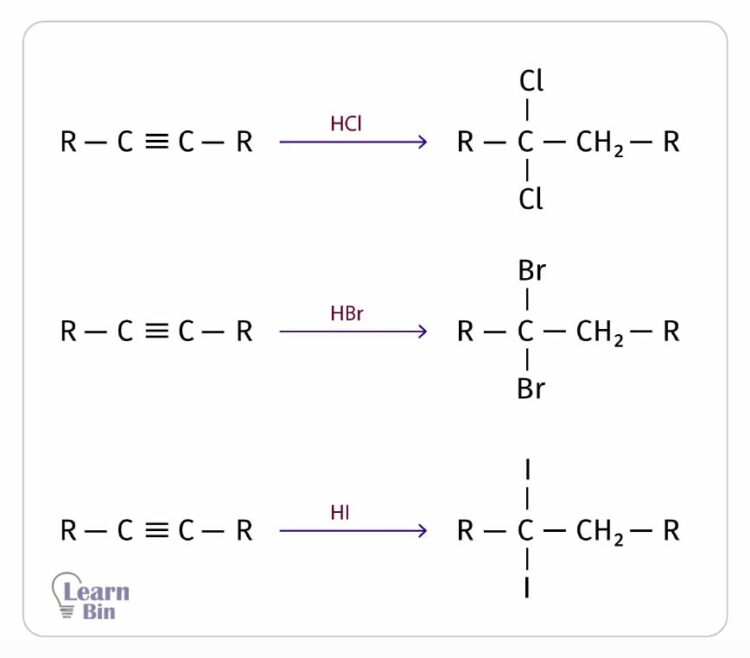
Alkynes react with hydrogen halides such as HCl, HBr, and HI and result in the respective alkyl halide. This reaction also follows Markovnikov’s rule. The carbon with more hydrogen gets the hydrogen atom, and the other carbon gets the halide.
For 1 mol of triple bonds, 2 mol of HX (X = Cl, Br, I) are added. Both halogens are attached to the same carbon atom.
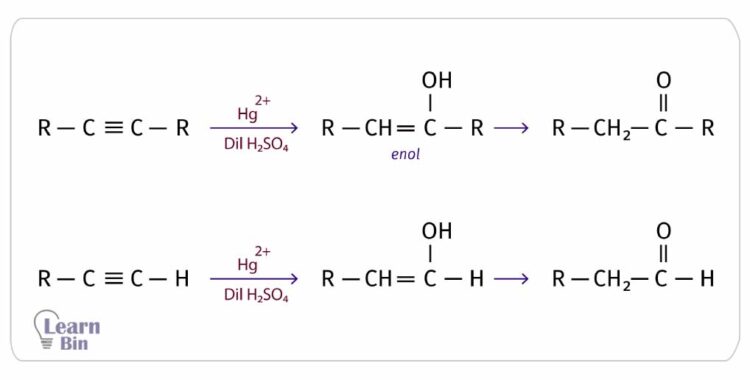
When an alkyne is heated with Hg2+ catalysts in the presence of dilute sulfuric acid, one mole of water molecules is added per one mole of triple bonds in the alkyne. It forms an enol that has a C=C double bond and an -OH group attached to one carbon atom in the double bond. However, enols are unstable, so they will rearrange into a stable keto compound (aldehyde or ketone).
An aldehyde results from only ethyne. All the other alkynes will result in ketones.
The two carbon atoms that are included in the triple bond are sp-hybridized. Sp hybridized orbitals have higher s characters (50%), and they are smaller when compared to sp2 and sp3 hybridized orbitals. So, the bond length of a C-C triple bond is less than a C-C double bond or a single bond.
Since the bond length is low, the electrons in the bond are closer to the nucleus. That means the electrons (negatively charged) on the carbon atom are closer to the positively charged nucleus. Therefore, the electronegativity of a sp-hybridized carbon is high. It can bear a negative charge easily.
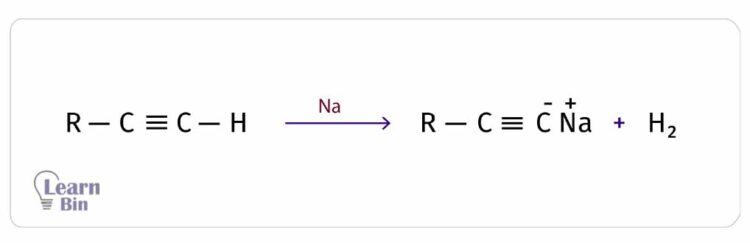
When there is a triple bond at the terminal carbon atoms, the Hydrogen attached to the terminal carbon can emit as an H+ ion, leaving a negative charge on the terminal carbon. Therefore, an alkyne with a terminal hydrogen has acidic properties. That means alkynes could react with metals and bases.
However, the acidity of terminal alkynes is very low when compared to the inorganic acids. Also, the acidity of terminal alkynes is even lower than water and alcohol. They could only react with highly reactive metals like sodium (Na) and strong bases like NaNH2.
Terminal alkynes result in the salt of the acetylide ion and H2 as products, from the reactions with the above reactants. In these reactions, the terminal hydrogen is substituted by the Na+ ion. Therefore, this reaction is an electrophilic substitution reaction.
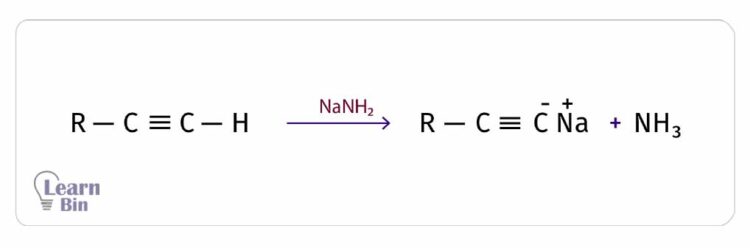
In some instances, terminal alkynes form insoluble metal acetylides. Those reactions can be used to identify terminal alkynes.
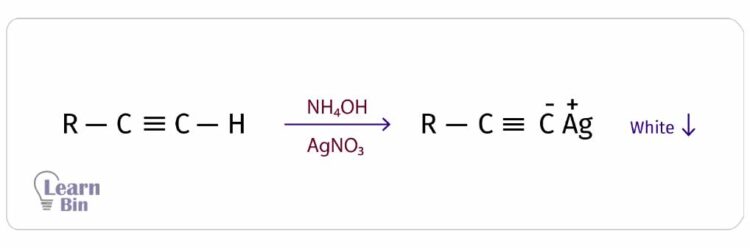
Terminal alkynes react with ammoniated silver nitrate (NH4OH + AgNO3) and result in a white precipitate of the metal acetylide. The acidic hydrogen is substituted by the Ag+ ion.
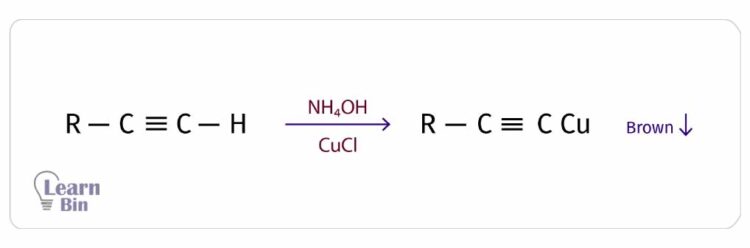
Terminal alkynes react with ammoniated copper chloride (NH4OH + CuCl) and result in a red/brown color precipitate of the metal acetylide. The acidic hydrogen is substituted by a Cu+ ion.

The cover image was created using the molecular editor from Molview.org
Figure 01: Image was created using the molecular editor from Molview.org