More results...
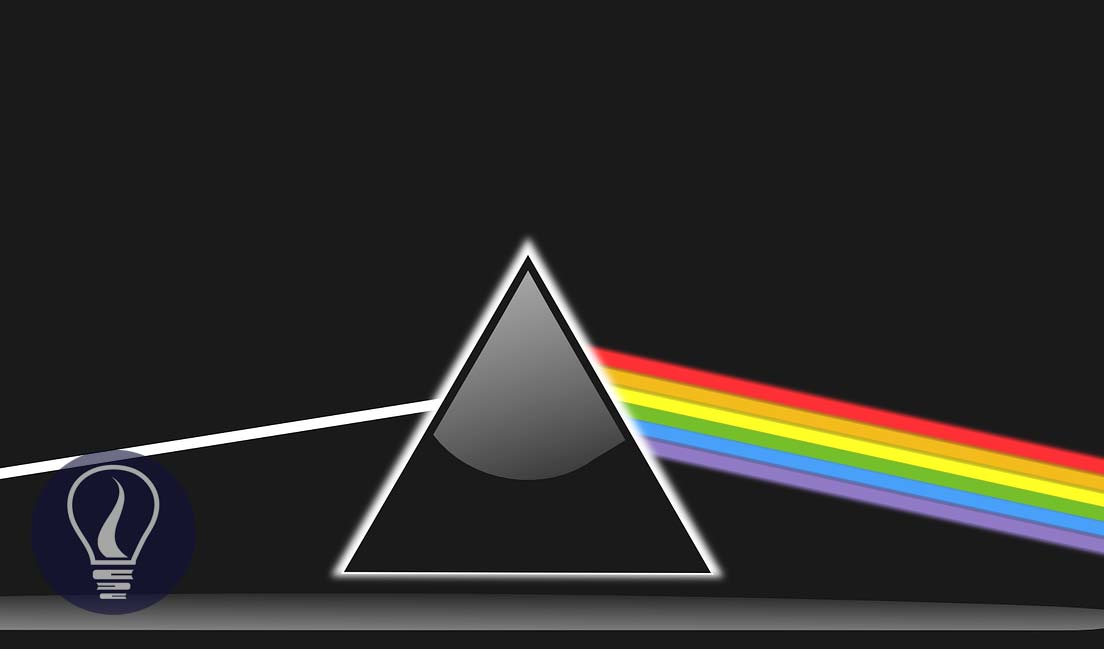

Simply, spectroscopy explains the interaction between electromagnetic radiation (Ultraviolet, visible light, infrared and etc.) and matter.
Spectroscopy is the studying of the interactions between matter and electromagnetic radiation and studying of absorbance characteristics and behavior of matter when matter interacted with electromagnetic radiation. In spectroscopic methods, samples (molecules/ matter) are exposed to a beam of electromagnetic radiation to obtain a spectrum.
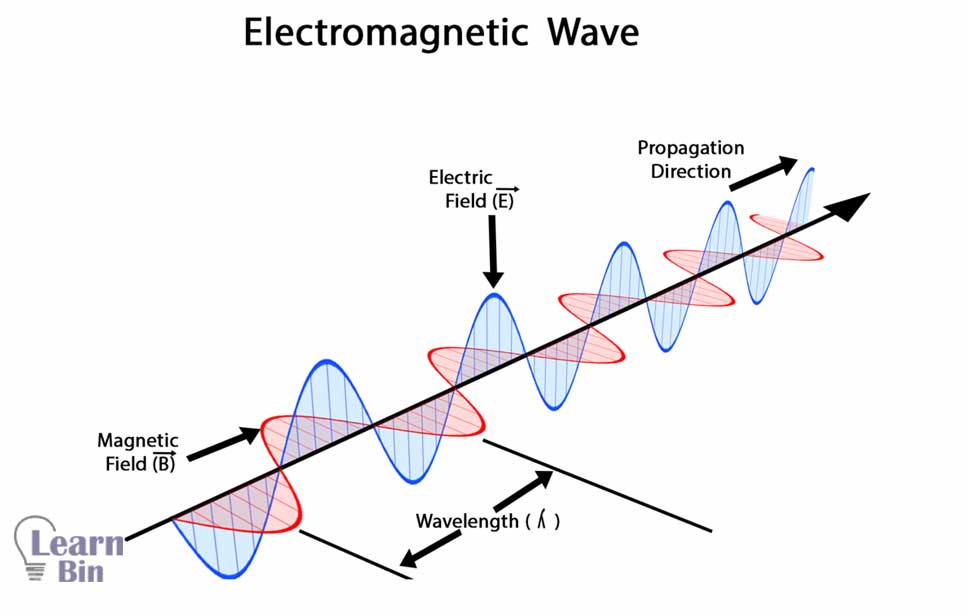
Spectroscopic methods can be used to identify molecules, their active groups, their structures, arrangements of the active groups, etc.
The concepts and principles of these spectroscopic methods will be discussed more deeply in separate articles.
Considering the kind of method used to get the spectrum, spectroscopy can be categorized into three types.
In absorption spectroscopic methods, the amount of radiation that was absorbed by the sample was used to determine the spectrum. For this, the instrument measures the intensity of the EM radiation before and after it interacted with the sample. Using this amount of absorption can be calculated.
Examples of absorption spectroscopic methods
Emission spectroscopic methods use electromagnetic radiations that were emitted by the sample to obtain the spectrum.
Examples of emission spectroscopic methods
When EM radiation passthrough molecules, a fraction of EM radiation scatter in a different direction due to vibrations of the molecules. This scattered EM beam has a different wavelength than the incident beam. This difference in wavelength depends on the structure of the molecule.
Examples of scattering spectroscopic methods
Determination of the nature of a material (molecule, substance, compound) using a quantitative measurement of an observable property like the amount of emitted light, the rate/ speed of the ions that move through a channel or medium, mass-to-charge ratio, can be identified as spectrometry.
Most of the time molecules are bombarded with high-energy electron (electric) beams to break them into ions. So high-energy electron beam is used to get the spectrum in spectrometric methods instead of electromagnetic radiation like in spectroscopic methods.
Examples for spectrometry
Spectroscopic methods use electromagnetic radiation to obtain a spectrum from a sample while spectrometric methods use energy sources like high-energy electric beams to obtain a spectrum from a sample. This is a key difference between spectroscopy and spectrometry.
Also, spectroscopy is a non-destructive method that means the radiation (energy) source won’t destroy (affect) the sample. But spectrometry is a destructive method because it involves breaking samples/molecules into ions/ fragments using high energy sources.
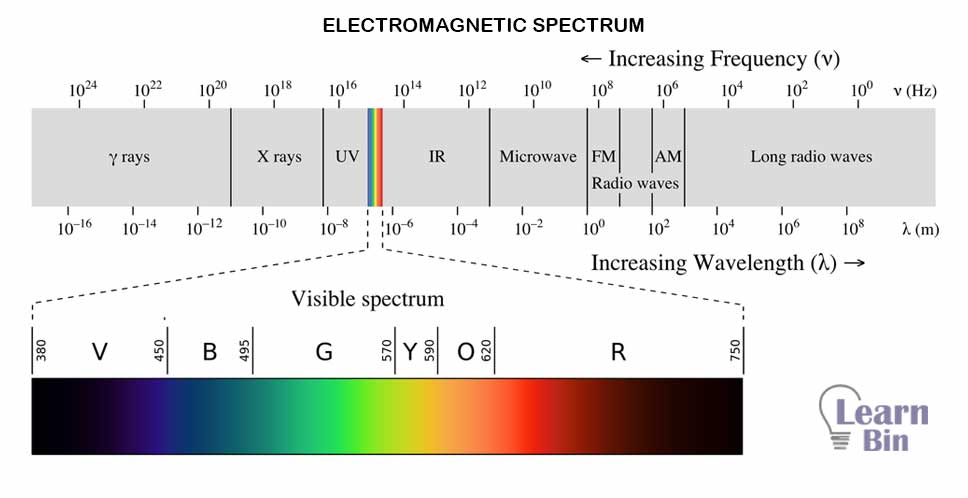
Different ranges of EM radiations interacted differently with the same molecule. So, the different wavelengths of EM radiations show different interactions in the molecules. That is because EM radiations that have high wavelengths, give low energy to a molecule. Also, EM radiations have lower wavelengths, giving higher energy to a molecule. That is why molecules show different behavior when they are subjected to different wavelengths of EM radiation.
E = hC/λ
It can be seen that energy is inversely proportional to the wavelength of radiation.
When electromagnetic radiation provides energy to a molecule (hits a molecule), the molecule has the ability to
When electrons present in a molecule or an atom absorbs energy from electromagnetic radiations, electrons are excited to the excited state (high energy) from the ground state (low energy).
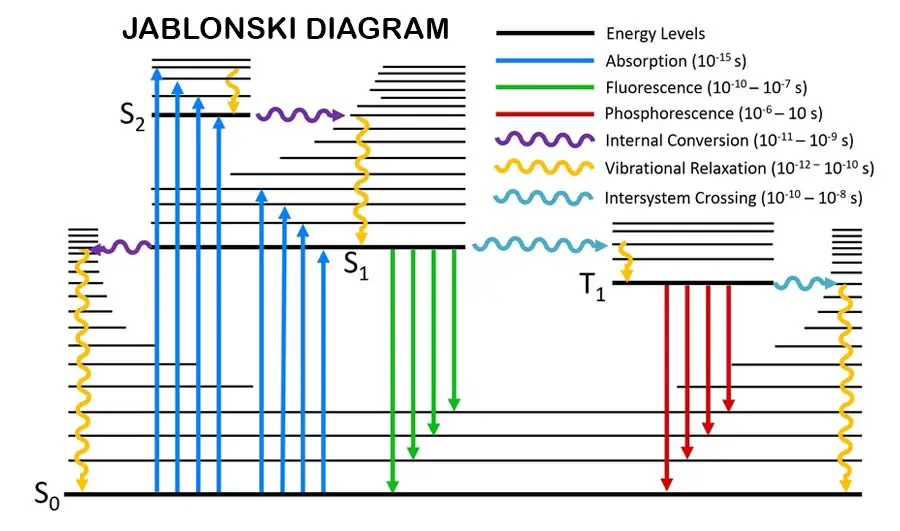
After that, those excited electrons have emitted that energy as electromagnetic radiation and fall back to the ground state.
When the above phenomenon happens because molecules interacted with electromagnetic radiation, that will cause some changes in the molecules. Some of those changes are causing rotational and vibrational motions in the molecules, causing lamination, and breaking bonds in the molecules.
For example, microwaves cause rotation in molecules. IR radiation cause vibrational motions in molecules. Visible light and UV could cause luminance and fluorescence in molecules. Also, UV radiation and X-ray could cause breaking bonds due to high energy.
This table shows different kinds of interactions that can be observed when molecules/ atoms are subjected to different EM radiation wavelengths.
| Type of EM radiation | Type of spectroscopy | Wavelength range | Type of quantum transition |
| Gamma radiation | Gamma-ray emission | Less than 0.01nm (1*10-11m) | Nuclear level transition |
| X-ray | X-ray emission, absorption, diffraction fluorescence | 0.01nm - 10nm (1*10-11m - 10*10-9m) | Inner electrons |
| Ultraviolet radiation | Vacuum UV absorption | 10nm – 180nm (1*10-8m – 18*10-8m) | Bonding electrons |
| Visible light and Ultraviolet radiation | UV- Visible absorption and emission spectroscopy | 180nm – 780nm (18*10-8m – 78*10-8m) | Bonding electrons |
| Infrared radiation | Infrared spectroscopy | 780nm - 300µm (7.8*10-7m – 3*10-4m) | Rotational and transitional energy levels |
| Raman spectroscopy | Rotational and transitional energy levels | ||
| Microwaves | Microwave absorption | 0.75mm – 3.75mm (7.5*10-4m – 3.75*10-3m) | Rotation of the molecules |
| Radio waves | Electron spin resonance (ESR) | 3cm (3*10-2m) | Spin of electrons |
| Nuclear magnetic resonance (NMR) | 0.6m – 10m | Spin of nuclei |

Khan Academy - Spectroscopy
Hollas, J., 2004. Modern spectroscopy. New York: J. Wiley.
Pavia, D., 2015. Introduction to spectroscopy. Stamford
The cover image was created using Image by OpenClipart-Vectors from Pixabay
Figure 01: DECHAMMAKL, CC BY-SA 4.0, via Wikimedia Commons
Figure 02: Philip Ronan, Gringer, CC BY-SA 3.0, via Wikimedia Commons
very well done