More results...

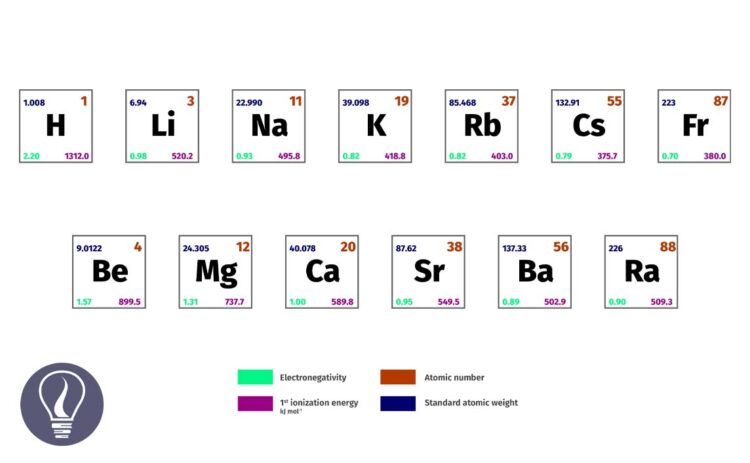
Except Hydrogen (H), Helium (He), and Beryllium (Be) all the elements in s block are metals. Helium is considered a noble gas (so helium is a group 18 element) and Beryllium possesses both metallic and non-metallic properties so, it is an amphoteric element. In this article, we are going to discuss the reactions of group 1 elements.
Group 1 elements are more highly reactive than group 2 elements due to the lower 1st ionization energy of the group 1 elements. This makes group 1 elements remove valance electrons easily and make +1 charged ions easily. Since the 1st ionization energy decreases downwards the group, the reactivity of the elements increases.
Group 1 elements cannot be stored in atmospheric conditions because they will react with Oxygen (O2) and moisture in the atmosphere vigorously. All the group 1 elements react with atmospheric Oxygen and result in their respective oxides.
The reactivity with O2 is increasing downwards the group. Lithium shows a relatively slow reaction with Oxygen. Therefore, it should be heated. Elements from sodium (Na) to Cesium (Cs) result in oxides without being heated.
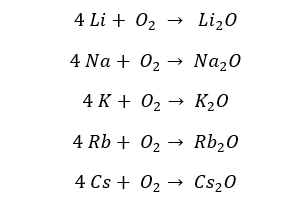
With the excess of Oxygen, elements from Sodium to Cesium result in peroxides. Sodium should be heated in the presence of excess Oxygen in order to result in peroxides. Lithium does not result in peroxides at the excess of Oxygen. In peroxides, Oxygen possesses a -1 oxidation state.
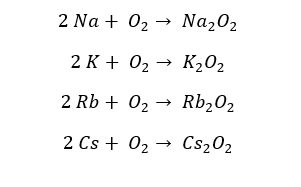
When Potassium is heated with excess oxygen, it will result in a superoxide as the main product. Rubidium and Cesium result in superoxides without being heated. In superoxides, Oxygen possesses -1 and 0 oxidation states.
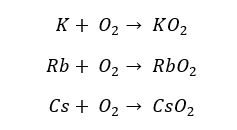
Resulted oxides, peroxides, and superoxides are highly alkaline. So, they will react with water as follows.
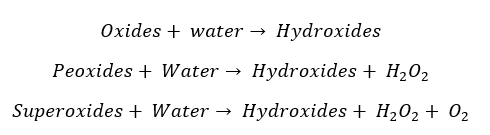
Except Lithium, group 1 elements do not react with Nitrogen. Therefore, group 1 elements from sodium to Cesium, are stored in a Nitrogen atmosphere. However, Lithium reacts with Nitrogen and results in Lithium nitride. Lithium nitride can further react with water and result in Ammonia gas.
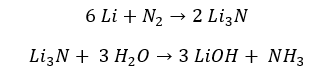
All the group 1 elements react with Hydrogen above 300 °C and result in the respective hydride. In these hydrides, Hydrogen possesses a -1 Oxidation state. The resulting hydrides are highly alkaline.
Therefore, they will react with water and result highly alkaline solution. The reactivity with water and the alkalinity of the given solution is increasing from top to bottom of the group.

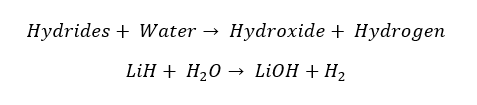
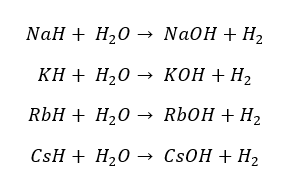
All the group 1 elements react with Chlorine and produce the respective chlorides. The chlorides of group 1 elements are neutral.
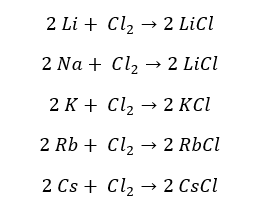
Except for Lithium, the group 1 elements react with water vigorously and result in the respective hydroxide and hydrogen gas. The reactivity with water increases when going down the group.
Lithium slowly reacts with water, when it is heated. Sodium reacts with cold water vigorously. Potassium reacts with cold water vigorously with an ignition. Rubidium and Cesium react with cold water explosively.
Except for Lithium, all the other reactions are exothermic. The resulting solution is highly alkaline.
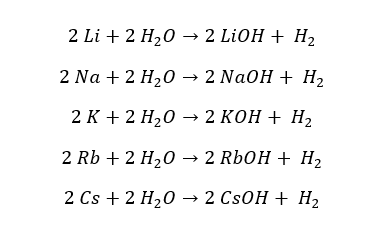
Group 1 elements react with diluted acids vigorously. These reactions produce the respective salt and hydrogen gas. All the reactions are exothermic.
Reactivity increases down the group. As an example, Sodium metal reacts with Hydrochloric acid (HCl) vigorously with an explosion and produces Sodium Chloride and hydrogen gas. From Sodium to Cesium, the reactions would be explosive.
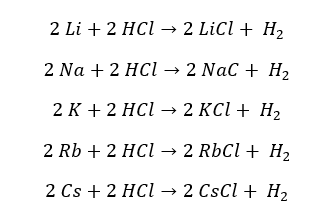

National Institutes of Health | PubChem - Periodic Table of Elements