More results...


Rubber vulcanization is the process that increases the retractile force and reduces the permanent deformation remaining after removal of the deforming force by the formation of the crosslinks. Crosslinks can be a group of sulfur atoms in a short-chain or, a single sulfur atom or, C-C bond or a polyvalent organic radical or ionic cluster or a polyvalent metal ion. After vulcanization, elasticity, strength, stability will increase while plasticity, tackiness, heat sensitivity decrease.
Vulcanizing agents which form the crosslinks between rubber chins are blended with the rubber in the compounding step. The compounded rubber next sends for rubber vulcanization and manufacturing final products.
The vulcanizing process should be done while or after giving the shape of the final product. Rubber has rubber properties after vulcanization it will not flow or melt in high temperatures. Therefore, the rubber cannot be shaped after vulcanizing.
Shaping techniques like calendaring and extrusion processes are used to manufacture sheet-like structures and ling uniform structures respectively. After shaping the product, it will be applied heat to be vulcanized.
When using molding techniques like compression molding, transfer molding, and injection molding, the compounded rubber will be vulcanized in the molding cavity. Because in such methods both heat and pressure will apply. In both processes, the compounded rubber is in a plastic state. After vulcanizing, it will become a rubbery state.
Sulfur is the most common vulcanizing agent used in rubber technology. In the natural rubber vulcanizing system, there are three components as sulfur as a vulcanizing agent, accelerator, and activator. Both insoluble and soluble sulfur is used as vulcanizing agents. Insoluble sulfur is much more expensive than soluble sulfur. But it uses to prevent unnecessary blooming in products.
There are three types of sulfur vulcanizing systems according to the amount of sulfur used for vulcanization.
In a conventional vulcanizing system, the sulfur/accelerator ratio is very high. A high amount of sulfur is used with low proportions of the accelerator. Therefore, it tends to form more polysulphidic crosslinks. Physical properties such as tensile strength, compression strength are good in the conventional system.
In this system, there is a high density of polysulphidic crosslinks. So, the compression set value is high. Because there are many ways to arrange molecules when applying a force. Due to the polymer network having mainly polysulphidic crosslinks which are thermally unstable, the rubber will have poor heat and aging resistance.
Contains a very little amount of sulfur. (0.4 Phr – 0.8 Phr). In this system, only mono-sulfidic crosslinks are formed. Rubber which is vulcanized by efficient vulcanization has a low set value and slightly low mechanical strength. Due to the mono-sulfidic crosslinks, it has excellent oxidative and aging resistance. Excellent heat resistance. Due to the poor flex-fatigue resistance, this rubber is not suitable for dynamic applications.
The sulfur level is between conventional and efficient systems. In this system, elemental sulfur is not used. Instead, sulfur donating accelerators are used. E.g., TMTD – Tetramethylthiuram disulfide. These compounds are used to replace part or all of the elemental sulfur. Normally used to produce vulcanized products containing fewer sulfur toms per cross-link.
| Vulcanizing system | Sulfur (Phr) | Accelerator (Phr) |
| Conventional | 2.0-3.5 | 0.4-1.2 |
| Efficient | 0.4-0.8 | 2-5 |
| Semi efficient | 0.8-2.0 | 1.2-2.0 |
Conventional, efficient, and semi-efficient vulcanizing systems are based on sulfur/ accelerator ratio, and it is applicable only for natural rubber, isoprene, and butadiene rubber.
Peroxide vulcanization is a radical process that can be used to vulcanize both saturated and unsaturated rubbers. This results in carbon-carbon crosslinks between rubber chains.
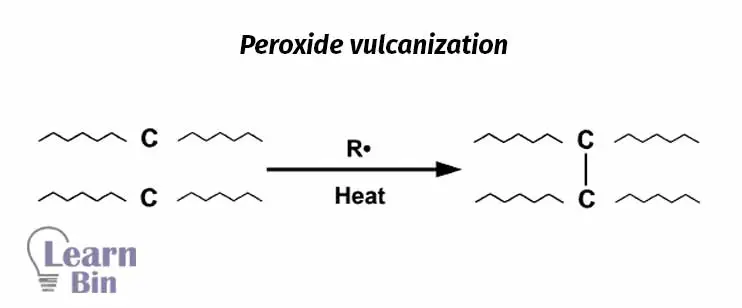
Some difunctional molecules form crosslinks by reacting with two rubber molecules. This result crosslinks with high thermal stability. Epoxy resins, phenolic resins with para alkyl substitutes can be used for resin vulcanization. However, resin vulcanization is a very slow process even at high temperatures. Therefore, the addition of catalysts is a must.
E.g. FeCl3, SnCl2
Non-olefinic rubbers like chloroprene cannot be vulcanized by sulfur because the Chlorine atom gives a hindered effect to the double bond of the rubber. In such systems, metal oxides are used as vulcanizing agents. A commonly used metal oxide is ZnO. It can act as both vulcanizing agent and vulcanizing activator.
Rheography indicates the variation of torque value of the rubber during the vulcanization. The compounded rubber was next filled into the mold cavity. In the mold cavity rubber is applied with both heat and pressure. Therefore, both shaping and vulcanization are occurring in the mold cavity.
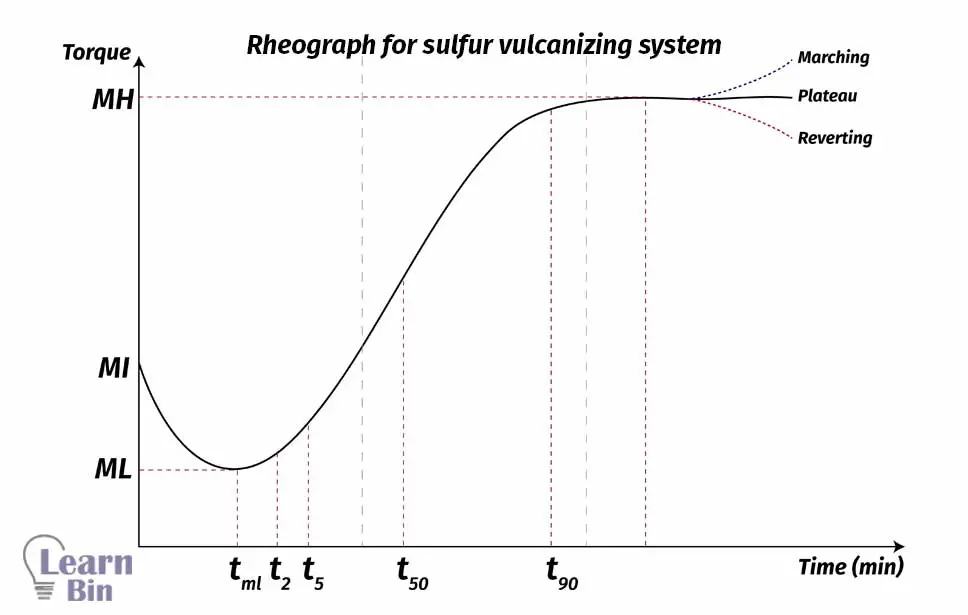
Torque values
Time values
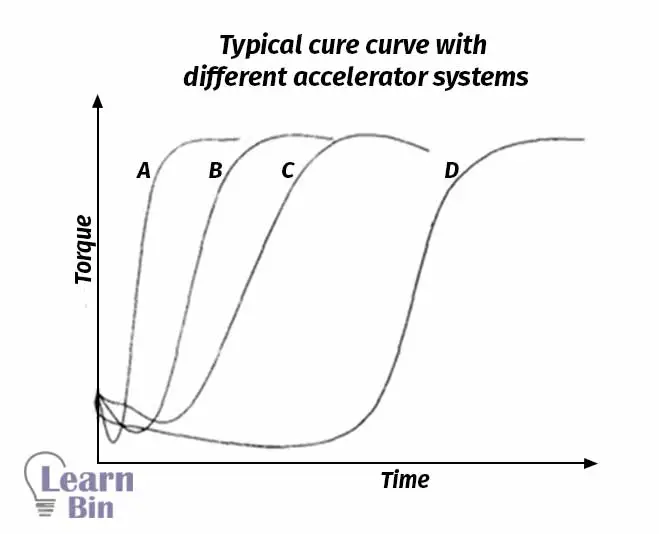

nocil.com - Vulcanization & Accelerators
The cover image was created using Image by webentwicklerin from Pixabay