More results...
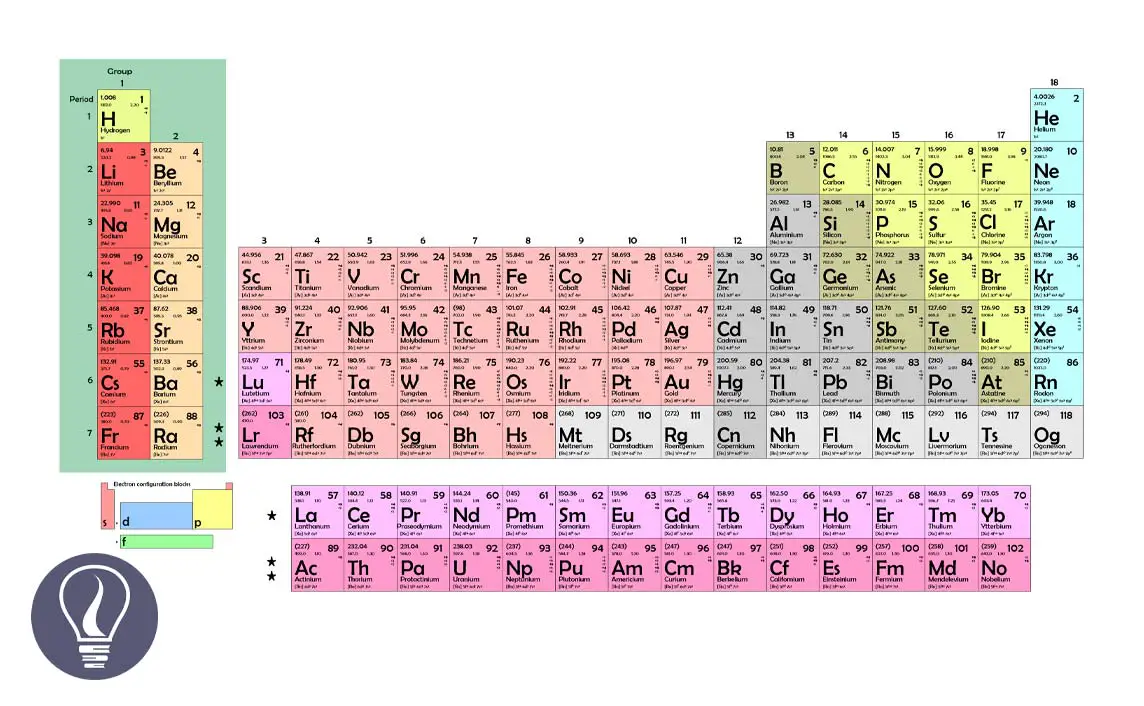

Elements in the periodic table are categorized into four groups of s, p, d, and f according to their electron configuration. When categorizing elements, we consider the subshell that is filled by the last electron of the element.
If the last electron is filled into an s subshell, the element is categorized into an s block. Elements are categorized into p,d, and f blocks when the last electron is filled into p, d, and f subshells respectively.
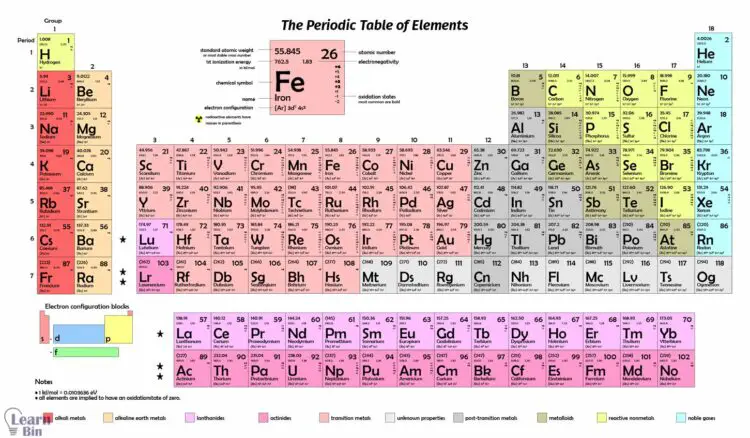
The elements in 1 and 2 groups of the periodic table belong to s block elements. There are seven alkali metals and seven alkali earth metals that belong to 1 and 2 groups of the periodic table comprise s block.
These are,
| Element | Atomic number | Electron configuration |
| H | 1 | 1s1 |
| Li | 3 | 1s2 2s1 |
| Na | 11 | 1s2 2s2 2p6 3s1 |
| K | 19 | 1s2 2s2 2p6 3s2 3p6 4s1 |
| Rb | 37 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 |
| Cs | 55 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 |
| Fr | 87 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 6f14 5d10 6p6 7s1 |
The number of electrons in the last subshell of the 1st group of elements is 1. By considering the last subshell, the common electron configuration for group 1 elements can be written as ns1. "n" can be 1, 2, 3, …
Electron configuration can also be expressed according to the electron configuration of a noble gas.
This means the electron configuration of Li, except the 1st subshell is similar to the electron configuration of He. So, the electron configuration of each alkali metal can be written as follows.
Among the elements in group 1, all the elements are metals except Hydrogen (H). Francium is rare and extremely radioactive.
Alkali metals remove one electron from the outermost shell and obtain the noble gas configuration. They make "+1 ion" and become stable.
Since Hydrogen has only one electron, either it can remove the electron or take an electron to obtain a noble gas configuration. Therefore, Hydrogen can obtain "-1 ion" as well.
When Hydrogen is bonded with an atom with higher electronegativity, Hydrogen shows a +1 oxidation state. When Hydrogen is bonded with atoms with lower electronegativity like Na, K, and Ca, Hydrogen shows a -1 oxidation state.
Except for Hydrogen, all the elements in group 1 show only a +1 oxidation state.
| Element | Atomic number | Electron configuration |
| He | 2 | 1s2 |
| Be | 4 | 1s2 2s2 |
| Mg | 12 | 1s2 2s2 2p6 3s2 |
| Ca | 20 | 1s2 2s2 2p6 3s2 3p6 4s2 |
| Sr | 38 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 |
| Ba | 56 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 |
| Ra | 88 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 6f14 5d10 6p6 7s2 |
The number of the electrons in the last subshell of group 2 elements is 2. Helium (He) comprises s block, but it has filled its outermost shell completely and become stable. Therefore, the properties of Helium do not match with group 2 elements. So, it has been considered a noble gas and is comprised of group 18.
Except for Beryllium (Be), all the elements in group 2 are metals. Beryllium is an amphoteric element. That means it shows both metallic and nonmetallic properties.
The elements in group 2 remove two electrons from their outermost shell and make +2 ions to stabilize. The valency of group 2 elements is 2 and the oxidation state is +2.

Cover image and Figure 01: Contains an image by 2012rc, licensed under CC BY 3.0, via Wikimedia Commons