More results...


In today's world, fossil fuels are the primary source of energy. It fulfills more than 80% of the energy requirements of the world. However, the problem is that all fossil fuels will run out in the near future. It is estimated that all the fossil fuels we have will be depleted in 2060. And the population is also growing. Therefore, it is necessary to find alternative energy sources. So, it is better to introduce renewable and sustainable energy. Solar energy can be known as the best renewable and sustainable energy we have. So, today, so many countries are focusing on solar energy conversion.
The main advantage of solar energy is completely free and is environmentally friendly. Conversion of solar energy does not emit CO2 or fume into the environment. And it does not generate any noise.
But there are disadvantages also. The initial cost for the implementation of solar panels is high. Sunlight is not always available (at night, on rainy days, etc.). On the other hand, the energy that comes to the earth from the sun is diffused. Lots of electromagnetic radiation is scattered from the layers of the atmosphere.
01. ΔH should be positive.
To convert solar energy into electricity, first, solar energy should be absorbed. Therefore, the photochemical reaction is endothermic. So, ΔH is positive.
02. The process should be cyclic.
The photochemical reaction should be repeatable. The molecules that absorb solar energy can excite and become ground state repeatedly.
03. No side reactions.
If there are any side reactions, that means the absorbed energy is used for another reaction without coming back to the ground state. So, if there are any side reactions, the photochemical reaction will not be repeatable.
04. Should absorb the white range of EMR
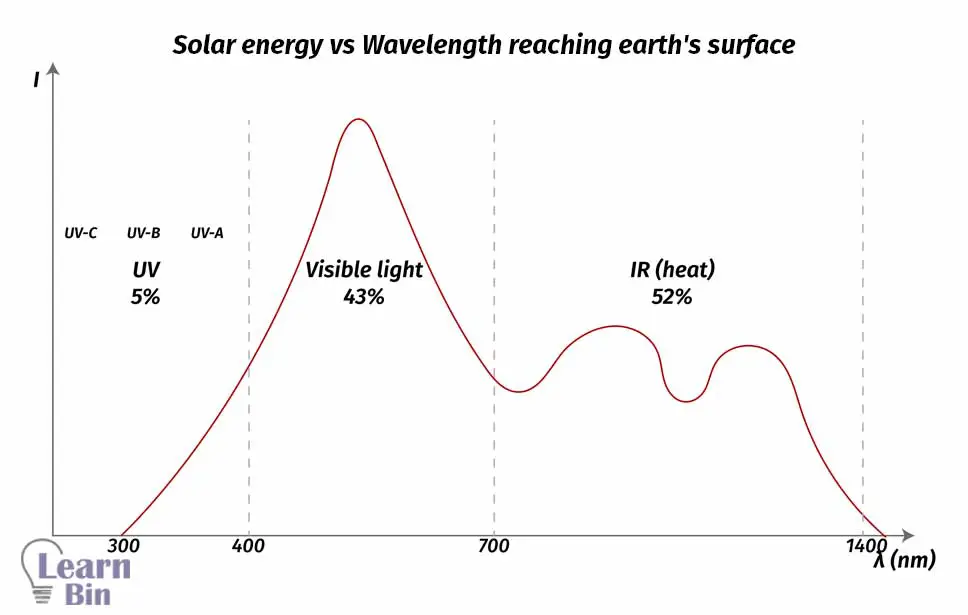
From the EMR that the sun produces, the Earth receives most of the IR and the white range of EMR (visible light). Earth receives 52% of IR, 43% visible light, and 5% of UV radiation. Due to the less energy of IR radiation, molecules will not undergo photochemical excitation under IR radiation. So, solar cell material should absorb the white range of EMR.
05. Quantum efficiency should be high.
To get higher requirements quantum efficiency of solar cells should be high. The Quantum efficiency of metals is about 4% - 10%. Because metals do not absorb all the radiation in the white range, even though metals are supplied with all the radiation in the white range, they will absorb a very narrow range of radiation.
Therefore, semiconductors like Si are used to manufacture solar cells. But theoretically, the maximum efficiency of Si solar cells is 29%. To increase efficiency, quantum dots, polymers, and nanoparticles are incorporated into the solar cells. These particles can absorb more range of the EMR in the white range.
To understand the conductivity of elements, it is important to understand their structures. The energy band model describes the structure of elements, and it can be used to understand the conductivity of elements.
According to molecular orbital theory, electrons in isolated atoms occupy atomic orbitals. When atoms form molecules, those atomic orbitals split into separate molecular orbitals with different energies. Bonding molecular orbitals have low energy, and antibonding molecular orbitals have high energy. When molecules form solids, there is a large number of molecules.
So, the bonding and antibonding orbitals form continuous energy bands. It forms two allowed bands that are valance band and the conductance band. The valance band is composed of bonding M.O., and it has low energy. The conductance band is composed of antibonding M.O, and it has high energy.
The valance band is always filled with electrons. The conductance band is not filled or partially filled with electrons. When electrons absorb energy, they move into the conductance band. Electrons in the conductance band are responsible for the conductivity of a solid.
Between the valance band and conductance band, there is a forbidden band, aka band gap. This band doesn't contain any electrons. The band gap can be decreased slightly by increasing the temperature.
In conductors like metals, the valance band and conductance band are overlapped. Therefore, electrons in the valance band can easily move to the conductance band without absorbing energy.
In some materials, the band gap is much higher. Therefore, electrons in the valance band need much higher energy to move to the conductance band. So, electrons can't move to the conductance band. Such materials are insulators.
In semiconductors, the band gap is intermediate. They should be provided little energy to electrons to move the conductance band from the valance band.
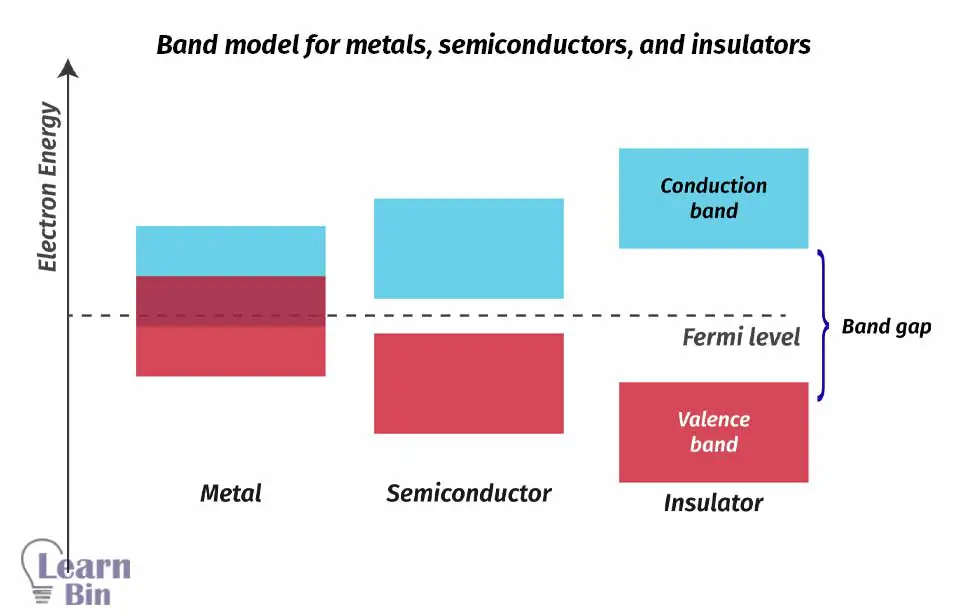
Considering the band model of solids, electrons can be excited and moved into the conduction band by supplying energy. Therefore, the material becomes a conductor that contains free electrons. This energy requirement can be fulfilled by solar radiation. This phenomenon can be explained by the photovoltaic effect.
When electromagnetic radiation hits a material, electrons are emitted. This phenomenon is called the photoelectric effect. Using this phenomenon, solar cells have been developed.
Photovoltaic cells are devices that can convert light energy into electricity. The first photovoltaic cell was invented by Edmond Becquerel in 1839. He submerged two electrodes in an electrolyte, and applied solar radiation. He observed that a voltage is generated when sunlight is applied.
Silicon solar cells are a type of photovoltaic cell where silicon is used to absorb solar energy. Si is used to absorb the energy of light. Si is a semiconductor, and it absorbs wavelengths that match the band gap of Si.
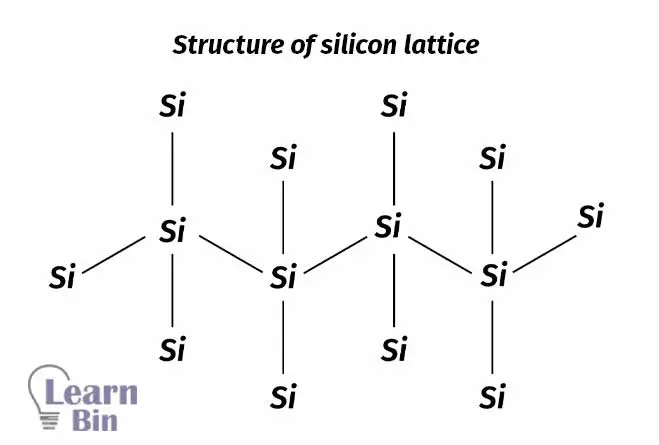
Electrons in Si-Si bonds will absorb the energy of light and move into the conductance band. These electrons in the conductance band will be responsible for the electric current flow. If there are more electrons in the conductance band, there is more current flow generated. So, Si is dope with an n-type or p-type dopant. Generally, 10% of dopant is dope with Silicon. Silicon solar cell is a PN junction of Silicon. A series of Silicon PN junctions are used as solar panels.
Advantages of silicon solar cells
Disadvantages of silicon solar cells
To overcome the disadvantages of silicon solar cells, Quantum dot solar cells have been introduced. Quantum dots are man-made semiconductors.
The efficiency of silicon solar cells is not much higher. All the sunlight on the device is not absorbed because Silicon only absorbs wavelengths that match its bandgap. Due to the reflection of radiation, some energy loses. Therefore, the theoretical efficiency of Si solar cells is 29%
Quantum dots are nanoscale particles that can absorb radiation in a wide range. Different sizes of quantum dots absorb different wavelengths of radiation. Incorporating quantum dots in solar cells can make solar cells absorb radiation in a wide range. So, the efficiency will increase.
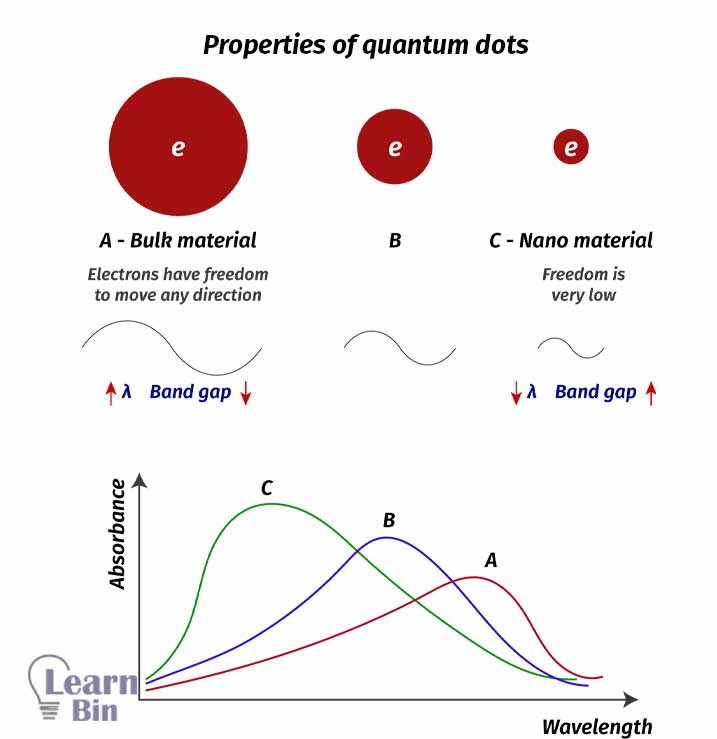
The size of a quantum dot directly affects the Wavelength that absorbs it. Electrons in smaller quantum particles have lower freedom to move. And they have a higher band gap. Therefore, smaller quantum dots absorb radiation with high energy (low wavelengths). Thus, larger quantum dots have lower band gaps and absorb radiation with lower energy (high wavelengths). By implementing quantum dots with different sizes, a wide range of radiation can be absorbed.
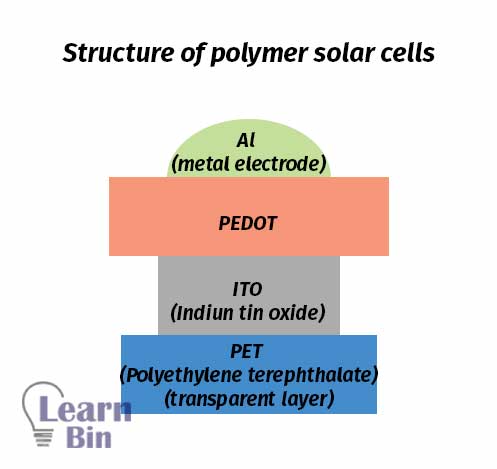
Polymer solar cells are a type of photovoltaic cell where conductive organic polymers or small organic molecules are used to absorb solar radiation. Conjugated systems in such molecules form a delocalized bonding π orbital with a π* antibonding orbital.
Delocalized bonding π orbital is the highest occupied molecular orbital (HOMO) which is considered the valance band. The delocalized π* antibonding orbital is the lowest unoccupied molecular orbital (LUMO) which is considered the valance band. The energy gap between HOMO and LUMO is taken as the band gap of the material.

The cover image was created using an Image by Sebastian Ganso from Pixabay