More results...


Polycarbonate (PC) is a high-performance heterogeneous polymeric material belonging to the family of "engineering thermoplastics." PC is one polyester. PC is a polymer that is a long-chain linear polyester of carbonic acid and dihydric phenols such as bisphenol-A.
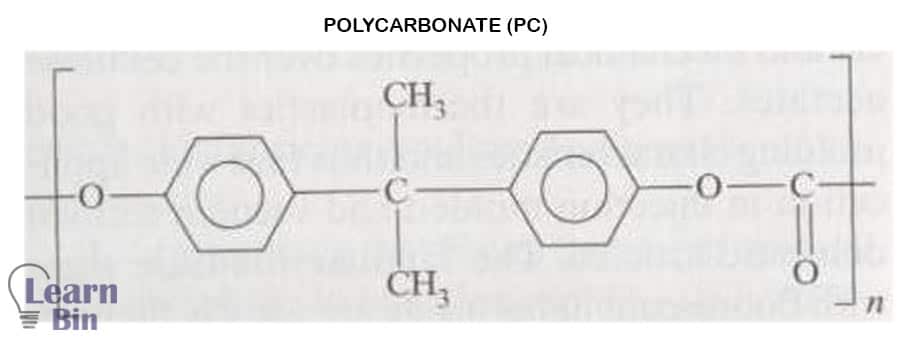
A PC molecule contains both a Bisphenol-A part and a carbonate group.
Bisphenol-A contains two aromatic rings. These aromatic rings increase the stiffness of the PC backbone. The Bisphenol A group is also responsible for PC’s inability to crystallize. This amorphous structure of polycarbonate gives the polymer special transparency.
Most often, polycarbonate is synthesized using Bisphenol A and phosgene using the step-growth polymerization method. In such a process chloride ions (Cl-) are eliminated every time when the monomers react. This kind of step-growth polymerization is usually called condensation polymerization.
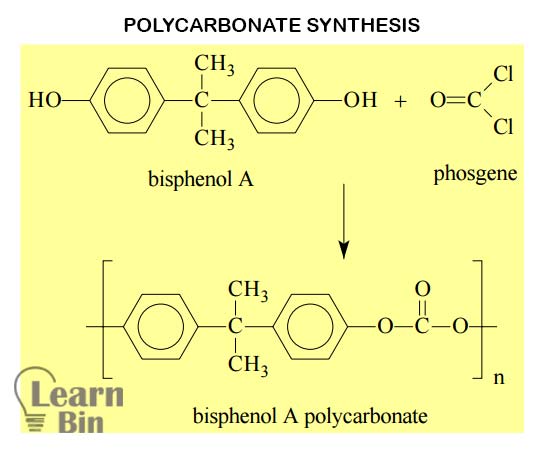
There are two methods of manufacturing polycarbonate industrially.
This process is also called the “Phosgene process”. This is a two-step process.
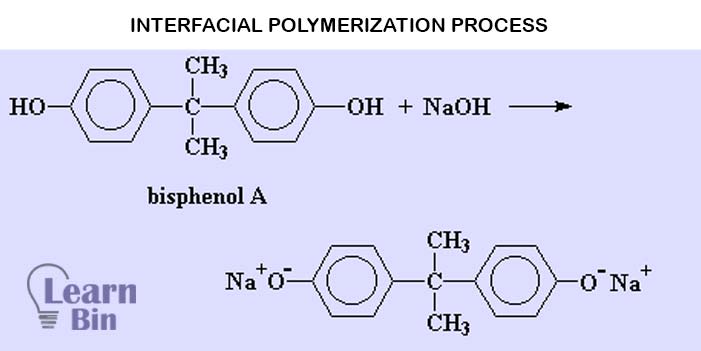
When synthesizing polycarbonate, the first step is to react 'bisphenol A' with sodium hydroxide to acquire the sodium salt of bisphenol A. The sodium salt of bisphenol A is then reacted with phosgene to produce the polycarbonate.
Here, the disodium salt of BPA reacts with phosgene dissolved in a chlorinated organic solvent such as CH2Cl2 (methylene chloride). This process results in high molecular weight PC. High molecular weight PC has excellent optical clarity and color. The major disadvantage of this process is that is a phosgene-based process.
In the melting process, BPA is condensed with diphenyl carbonate at elevated temperatures and at reduced pressure. The reaction is a base-catalyzed reaction. (hydroxides, hydrides, and amides of alkaline and alkaline earth metals and metal oxides such as zinc oxide and antimony oxide) This process results in low molecular weight polycarbonate below 30000g/mol.
The major advantages of this process are solvent-free and potentially phosgene-free.
Bisphenol A and the ester are heated together to obtain a molten mass of the polymer.
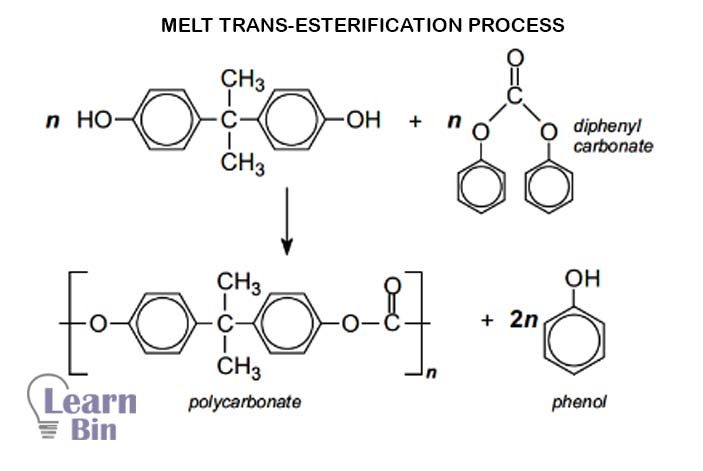
Phenol and excess reagents are removed by distillation under low pressure.
There are significant differences between these two processes that may make them advantageous and disadvantageous. The interfacial process is capital intensive to purify the resin solution, isolate and dry the resin, and recycle solvents.
With melt transesterification, the only recycling stream is the recovery of phenol for reuse. Hence, there is no need to invest in solvent recovery infrastructure with the melting process, and polymer purification units and dryers can be avoided.
The amorphous structure of PC offers outstanding optical properties. If there are impurities the polymer becomes yellow in color. If not, it is water white. Therefore, PC is able to be colored to form transparent, translucent, or opaque parts.
Due to the presence of polar groups, PC shows good mechanical properties over a broad temperature range. PC has a high melting point (270 - 300℃), high softening point (150-190℃), and high tensile strength of around 600-800 kg/cm2. PC shows outstanding impact strength even at low temperatures. And also, PC is a high creep resistive material.
There are no tertiary or secondary carbons in the PC structure. Therefore, PC has good temperature resistance. So, no need to add stabilizers. PC is stable at very high temperatures without destruction.
| T=300 ℃ | No oxidation |
| T=320 ℃ | Stable for many hours |
| T=330 ℃ | Stable for several minutes |
| T= and above 340 ℃ | destruction |
PC is also processed by traditional plastic processing methods.
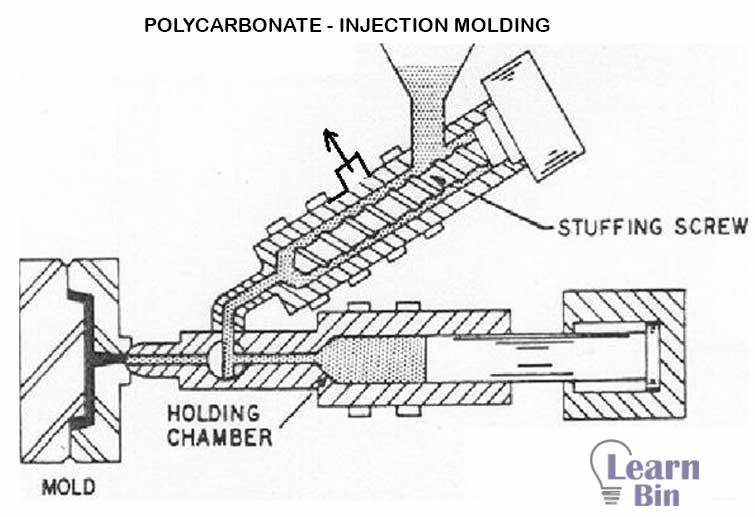
If PC was made by interfacial polymerization, it is recommended to use two-stage injection molding machines. PC with a molecular mass of around 32000-35000 g/mol can be processed by injection molding. The water percentage of PC should be below 0.01%. Therefore, to remove excess water PC is dried at 110-120℃ under a vacuum for several hours.
The processing temperature at the mixing chamber is 240-330℃. But, at the nozzle, the temperature is 350 ℃. The nozzle is open as this polymer is of high molecular weight. The temperature of the screw is maintained at around 230-290℃. It is not cooled. Because Polycarbonate strongly adheres to the metal if cooled. Even shrinkage may damage the metal.
Maximum injection pressure is 700-1000kg/cm2
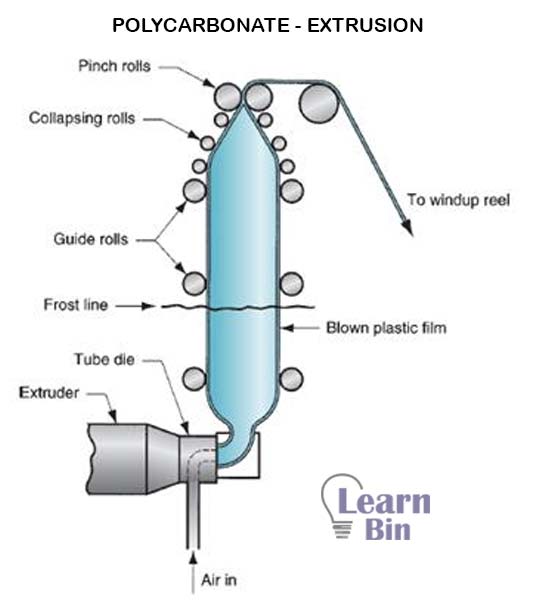
The extrusion process of PC results in three types of products. Those are thick sheets above 300 microns of thickness, thin films about 6 microns of thickness, and PC tubes. PC Films can be extruded as blown film and PC sheets by flat extrusion. PC with a molecular mass of around 30000 g/mol is used to manufacture films by extrusion blow molding process.
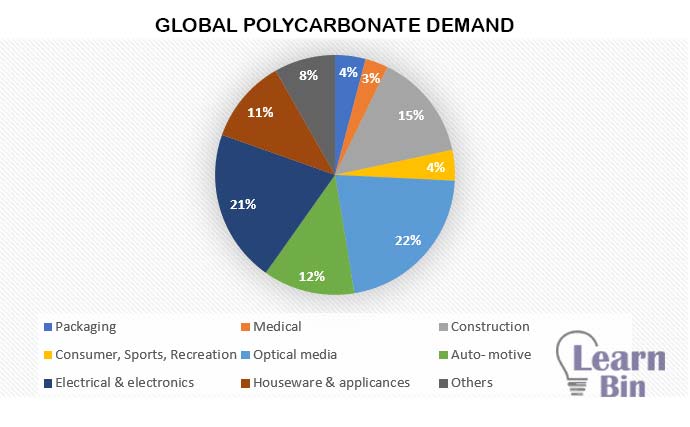
Polycarbonate is used in at least 27 major industries, segments ranging from telecommunications components to eyewear, to health care devices, to optical storage discs. Global Polycarbonate demand in 2015 is approximately 4.3 million tons. It is expected to reach 7.7 million tonnes in 2024.


Polycarbonates - The Essential Chemical Industry
Cover Image by Marlon Falcon Hernandez from Pixabay