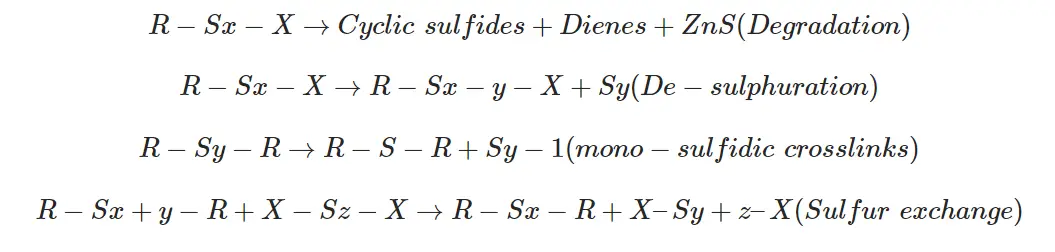More results...


Vulcanization is the process that forms crosslinks between rubber chains. The pre-vulcanization reaction takes place in each latex particle without altering its shape, and appearance. Pre- vulcanized latex is very similar to un-vulcanized latex in appearance. It is possible to pre-vulcanize the dispersed phase of natural rubber latex without any prior coagulation.
Necessary Compounding ingredients in a sulfur vulcanizing system are sulfur as the cross-linking agent, Activators, Accelerators, Stabilizers, pH builders, Fillers, Pigments, Thickeners, etc. Latex compound is prepared by dispersing these compounding agents with preserved natural rubber latex. latex compound can be either dispersion or emulsion.
| Ingredient | Parts by weight | |
| Dry | Wet | |
| NR latex (60%) | 100 | 167 |
| KOH (10%) | 0.3 | 3 |
| Stabilizer (10%) | 0.2 | 2 |
| Sulfur (50%) | 2 | 4 |
| ZnO (50%) | 1 | 2 |
| ZDEC (50%) | 1 | 2 |
Sulfur vulcanization of NR latex occurs at many low temperatures when compared to sulfur vulcanization of dry rubber. Sulfur vulcanization in the latex stage occurs much more rapidly than the vulcanization of dry rubber at the same temperature using the same vulcanizing ingredients.
Sulfur pre-vulcanized NR latex is slightly yellow. The density of latex increases with pre-vulcanization.
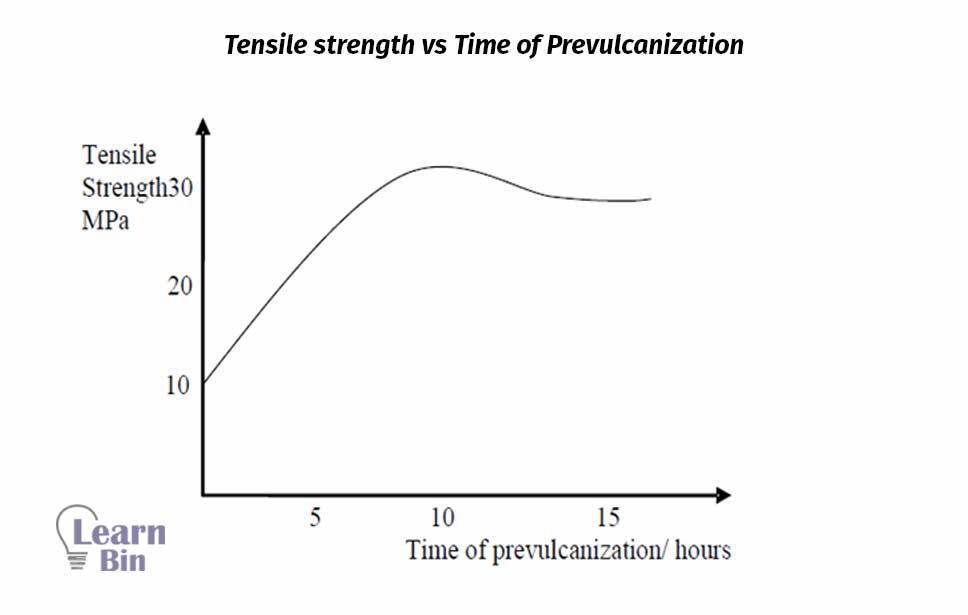
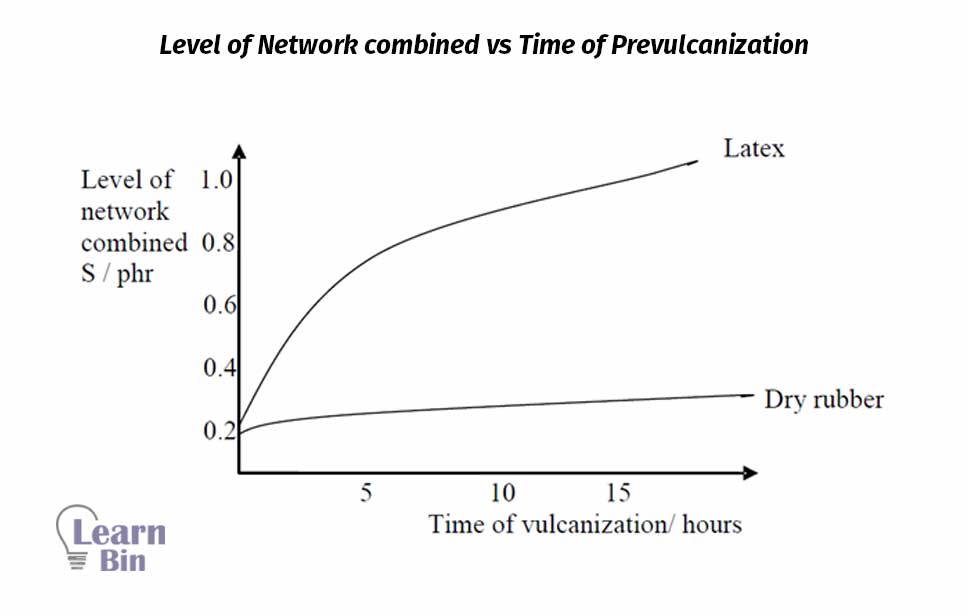
Similar results have been obtained for synthetic cis 1,4-polyisoprene. This indicates that 30% of the water in the latex may be responsible for the occurrence of sulfur pre-vulcanization at rather a low temperature.
It is assumed that the vulcanization reaction starts by a mechanism similar to the sulfur vulcanization of dry natural rubber.
First, the vulcanizing activator reacts with the S8 ring and forms an active sulphurating agent.
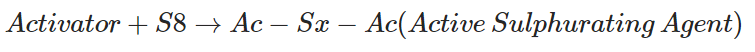
Active sulphurating agent reacts with natural rubber chains and forms activator + sulfur (Ac-Sx) pendent groups. (Polysulphidic pendant groups)
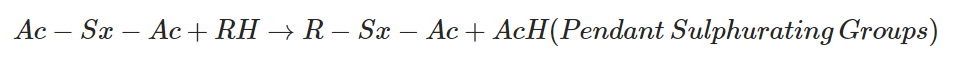
The polysulfidic pendent sulphurating group further reacts with another rubber chain and forms sulfur crosslinks. The activator is removed.
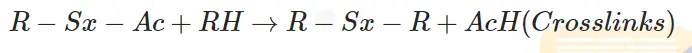
Some sulfur atoms are removed from pendent sulphurating groups. (De-sulphuration)
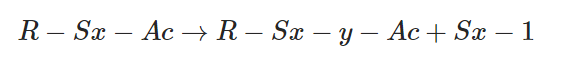
By removing sulfur atoms from polysulphidic crosslinks mono-sulfidic and di-sulfidic crosslinks are formed.
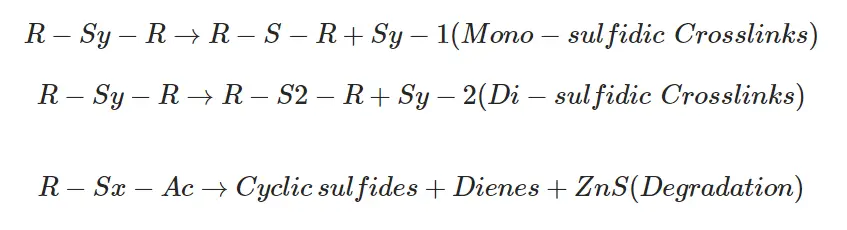
The rearrangement reactions are as follows.