More results...


Thermal decomposition is a chemical reaction that breaks down a chemical compound due to the heat energy. To occur the reaction the difference in Gibbs free energy (ΔG) should be a minus value. When the compound is heated the value of the |ΔG| is increased. That means the reaction is more favorable in high temperatures.
When talking about the thermal decomposition of s-block compounds, it is important to explain about the thermal stability. The thermal decomposition of a compound is based on its thermal stability.
All the S block elements produce ionic compounds. Usually, ionic compounds have higher thermal stability than covalent compounds. However, the ionic properties of the ionic compounds change according to the polarizability of the anion and the polarizing power of the cation in the ionic compound.
Positive and negative ions are formed when removing electrons and gaining electrons respectively. Ionic compounds are formed due to the electrostatic attraction between the positive and negative ions. (Cations and anions)
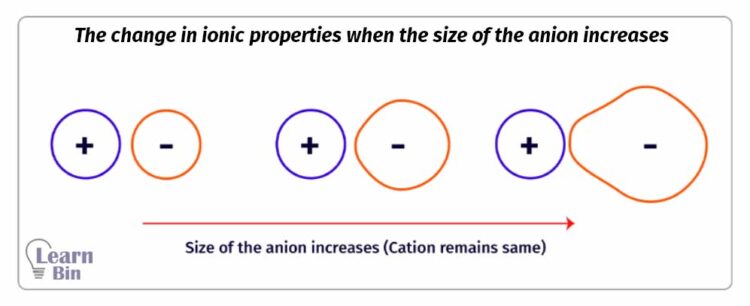
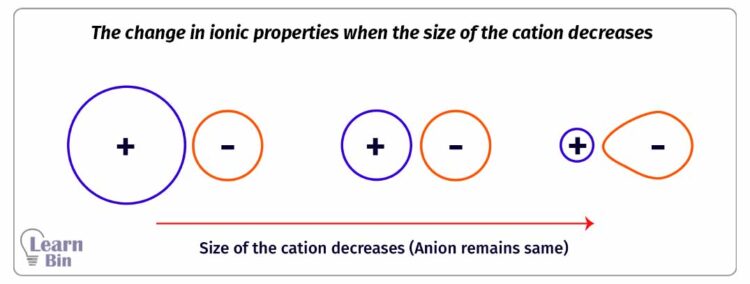
The Electron cloud of a cation is tightly bound to its nucleus hence cations are smaller in size comparatively. The electron cloud of an anion is loosely bound to its nucleus and anions are comparatively large in size. Therefore, the electron cloud of an anion can be distorted by an external electric field.
The tendency of attracting the electron cloud of the anion in an ionic compound by the cation is called the polarizing power of the cation. The polarizing power of a cation is increased when it is higher in charge and smaller in size.
Due to the polarizing power of the cation, the electron cloud of the anion will be distorted. The tendency of an anion to distort its electron cloud is known as the polarizability of an anion. The polarizability of an anion is increased when it is higher in charge and larger in size.
The ionic properties of an ionic compound are decreasing when the cation has high polarizing power and the anion has higher polarizability and vice versa.
The s-block elements, consisting of alkali metals (group 1) and alkaline earth metals (group 2), form compounds that exhibit unique thermal properties and behaviors due to the nature of their bond formation, and election configuration.
When provided with thermal energy, thermal decomposition of s-block compounds occurs and decomposes into simpler products, often releasing gases or forming oxides, carbonates, or other byproducts. Also, some of these s-block compounds are thermally stable and do not decompose under normal conditions.
Understanding the thermal decomposition reactions of these compounds is important in various industrial processes, from material synthesis to environmental applications. So let's explore the decomposition mechanisms, key reactions, and practical significance of common s-block compounds.
Except for Lithium nitrate (LiNO3), all the nitrates of group 1 elements decompose into their respective nitrites and release oxygen gas. Lithium nitrate decomposes to lithium oxide, releasing two gases which are nitrogen dioxide and oxygen.
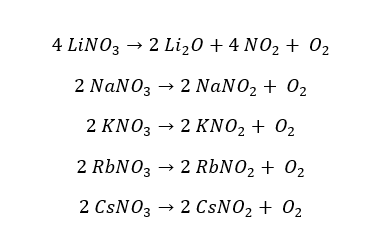
All the nitrates of the group 2 elements decompose into their respective oxide releasing oxygen gas and nitrogen dioxide gas.
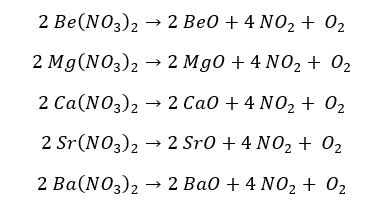
The thermal stability of nitrates increases down the group. Therefore, the heat energy that should be supplied for the decomposition is increased. This is because the ionic properties increase from the top to the bottom of the group.
Since the anion is the same in each nitrate, the polarizability of the anion remains the same. But the size of the cation increases down the group. Therefore, the polarizing power decreases. Thus, the ionic properties increase down the group.
From the carbonates of the group 1 elements, Lithium carbonate (Li2CO3) decomposes to Lithium oxide and releases Carbon dioxide gas. All the other group 1 carbonates are thermal stable.
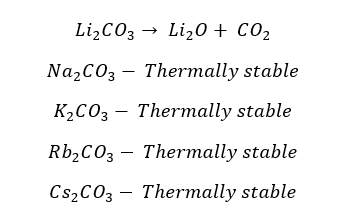
All the carbonates of group 2 elements decompose into their respective oxide and release carbon dioxide gas.
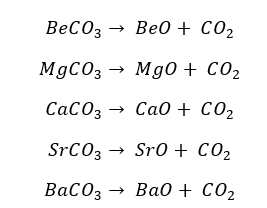
The thermal stability of carbonates increases down the group. Therefore, the heat energy that should be supplied for the decomposition is increased. This is because the ionic properties increase from the top to the bottom of the group.
Since the anion is the same in each carbonate, the polarizability of the anion remains the same. But the size of the cation increases down the group. Therefore, the polarizing power decreases. Thus, the ionic properties increase down the group.
When compared to the group 2 carbonates, group 1 carbonates are thermally stable. The carbonates of group 1 have larger cations than the group 2 carbonates. Also, the ionic charge is lower than that of group 2. Therefore, the polarizing power is low in group 1 cations.
Thus, the ionic properties are higher in group 1 carbonates compared to group 2 carbonates. Therefore, group 1 carbonates are more thermally stable.
All the bicarbonates (aka hydrogen carbonates) of s-block elements are thermally unstable. Group 1 bicarbonates decompose into their respective carbonates and release water and carbon dioxide gas.
Since Lithium carbonate is thermally unstable, it will further decompose into Lithium oxide, releasing carbon dioxide gas.
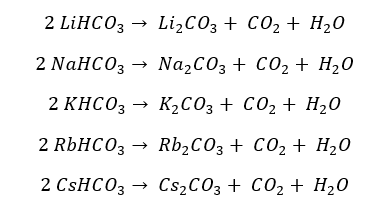
Most bicarbonates of group 2 elements exist in an aqueous state. When the heat is supplied, it will precipitate before the water is boiling.
After the water is completely evaporated the bicarbonates will decompose into their respective carbonates.
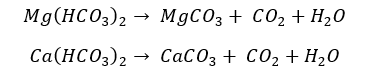

The cover image was created using an image from PxHere