More results...


A chemical reactor is an enclosed volume where a chemical reaction occurs in a chemical process. The most important part of a chemical plant is the chemical reactor.
A batch reactor is the simplest chemical reactor. It contains a reaction chamber and impeller for good mixing. First, the reactor is filled with reactants, and it is closed. Then the reaction is started. The reaction occurs in a closed system. Therefore, reactants cannot be added, or products cannot be removed during the operation. Products are removed as batches at the end of the process.
Impellers in batch mixers are responsible for the good mixing of the reaction medium. Therefore, the composition and the temperature are the same at any two places of the reaction medium. Also, the reaction rates and extent of reaction are the same at any two places at the given moment.
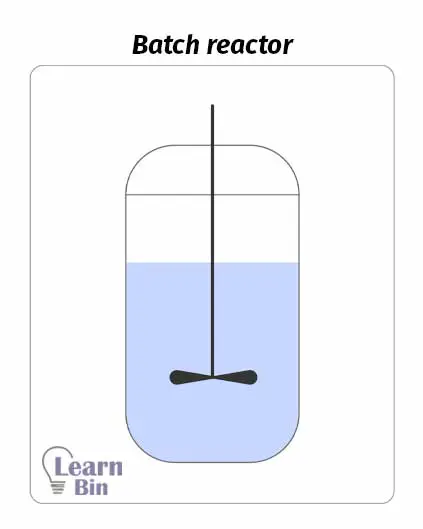
As the products result in batches, the batch mixer is suitable for small-scale production. If a reaction takes a very long time to complete, we can use a batch reactor. But this reactor is not suitable for large batch sizes.
As same in a batch reactor, a semi-batch reactor contains a reaction chamber and mixing impellers. But the difference is, in semi-batch reactors, we be partially filled with reactants and removed products during the process. As there are impellers, inside the reactor, there is good mixing. Good mixing leads to uniform composition and temperature in the reaction medium. Therefore, both batch and semi-batch reactors are very efficient.
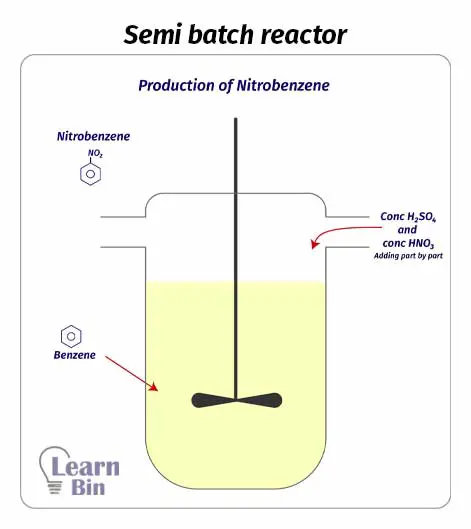
Due to the flexibility of adding reactants and removing products during the process, there is better temperature control in semi-batch reactors than in batch reactors. Therefore, this reactor is suitable for exothermic reactions.
On the other hand, removing products in the system increases the reaction rate of the forward reaction in an equilibrium reaction.
A continuous stirred tank reactor basically contains a reaction tank and an impeller. Reactants are continuously introduced to the reactor and products are continuously removed. Inside the reactor, there is well agitation and mixing. Therefore, in the concentration of incoming fluid (reactants) will instantaneously become the concentration inside the reactor.
This reactor operates at a steady state. That means the charging rate of reactants is equal to the discharging rate of the product. So, inside the reaction chamber, there is no accumulation of the product.
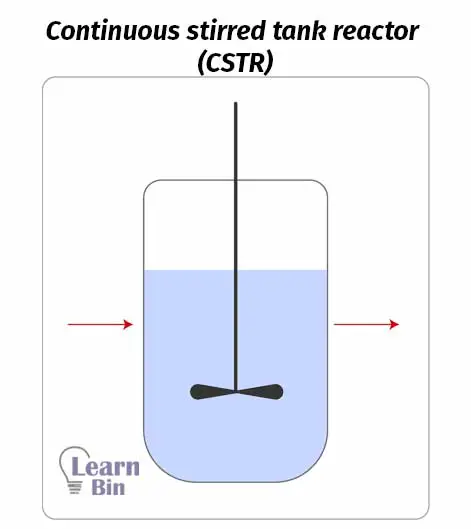
As we can get products continuously, this reactor is suitable for large-scale production. But the reaction should not be slow.
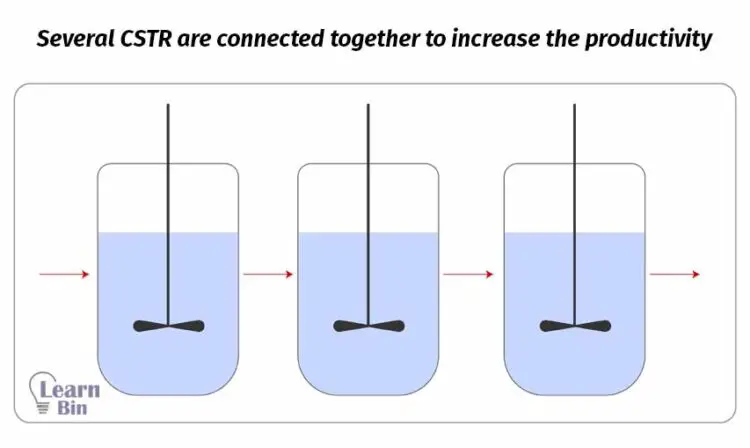
Sometimes, several continuous reactors are connected together in parallel or in series to increase productivity.
A plug flow reactor is a tube-like reactor. This is a modification of CSTR. Therefore, this reactor is also known as a continuous tubular reactor (CTR). In plug flow rectors there are no impellers and no mixing system inside the reactor. Therefore, the reactants should be mixed before sending to the reactor.
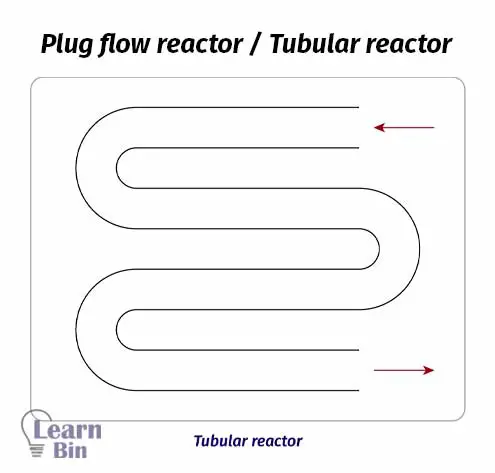
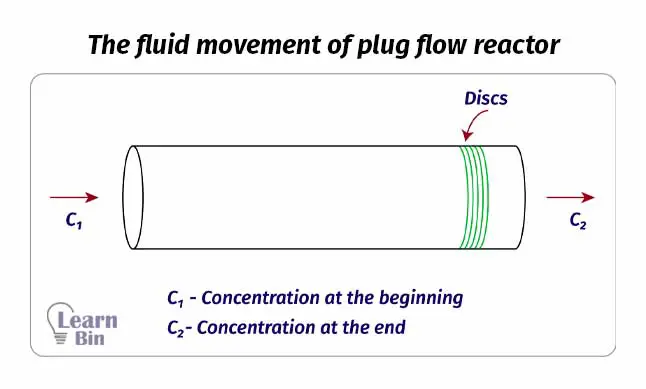
The reactant mixture is pumped through the tube of the reactor. While the reaction medium passes through the tube, the reaction occurs. At the end of the tube, we can get products. this reactor is also operated at a steady state as the continuous reactor. As there is no mixing system inside the tube, there is a concentration gradient.
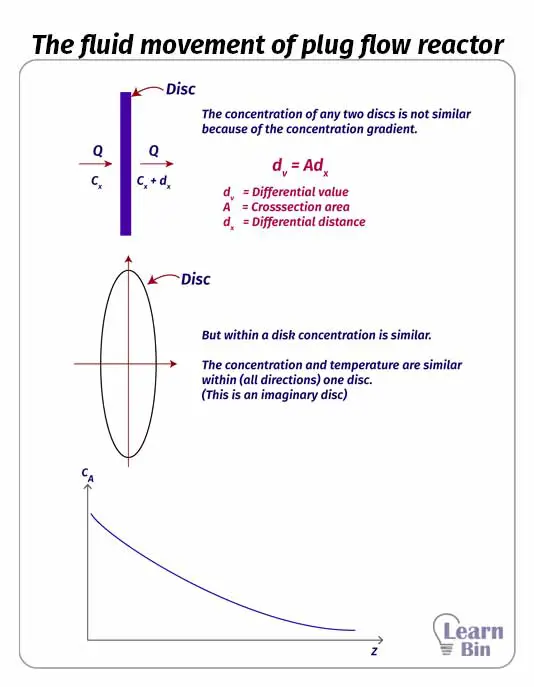
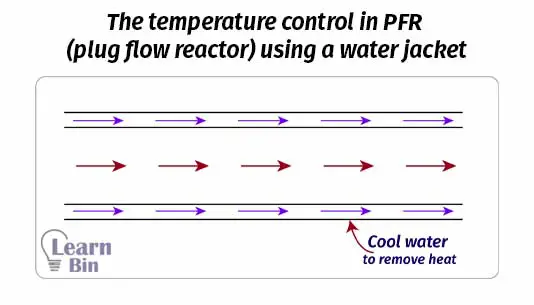
In PFR the reaction medium is passed through a long path. Therefore PFR has higher efficiency than CSTR of the same volume. We can control the temperature in PFR. If the reaction is exothermic, the reactor can be cooled by using water jackets.
To increase the flexibility of the reactor, it can be modified by adding several inlets other than the main inlet. Reagents or additives can be introduced at any location of the reactor, other than the main inlet.
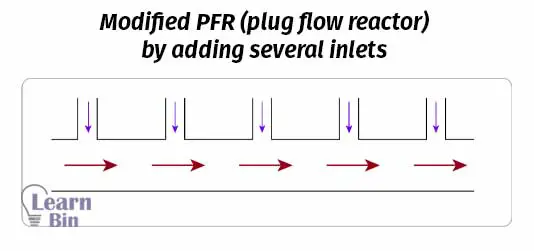
Sometimes PFR is connected to several pipes or tubes parallelly to increase the efficiency of the reactor. PFR can operate horizontally or vertically. So, we can manage the space.
Packed bed reactors or fixed bed reactors are mainly used for catalytic reactions. This reactor simply consists of a cylindrical shell with a convex head. There are no impellers in packed bed reactors. Solid catalysts are packed inside the reaction chamber And the reactants are introduced from the top of the reactor. Reactants flow through the immobile catalyst particles under gravity. Therefore, most PBRs are vertical.
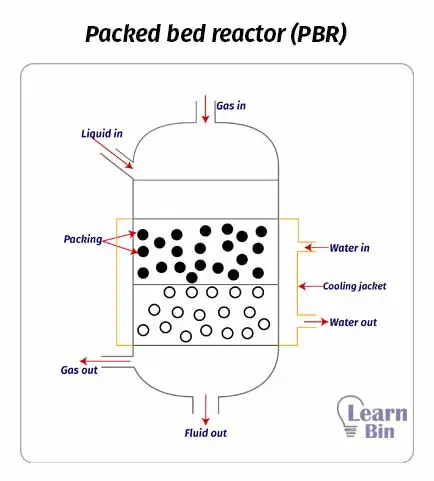
In PBR, the reaction medium has high contact with catalysts. Therefore, PBR results in a high yield per unit volume.
Due to the backed bed of catalysts, there is no mixing system in this reactor. So, it is difficult to control the temperature and a temperature gradient may occur. The other disadvantage of PBR is the difficulty of replacing catalysts.
In a fluidized bed reactor (FBR) a gas or liquid (fluid) is passed through a granular solid material at a high speed. This speed is high enough to make solid particles float in an upward direction. This process is known as fluidization. Usually, these solids are tiny spheres of catalysts.
Catalysts (solid particles) are placed on a pores plate which is called a distributor. Fluid is passed through the distributor at high velocity. When the fluid is passed at lower velocities, the solid particles will not float, and the fluid is just passed through the voids between solid particles. As the speed is increased, at a time the speed is enough to start floating the solid particles. This is the minimum speed at which to start the fluidization. This is the incipient fluidization of the system.
Once this minimum velocity has been exceeded, reactant content in the FBR begins to swirl through the reactor. Therefore, the mixing is taken place. So, there are no impellers in a fluidized bed reactor for mixing. At this stage, the reactor is a fluidized bed. FBR can be operated in a continuous state.
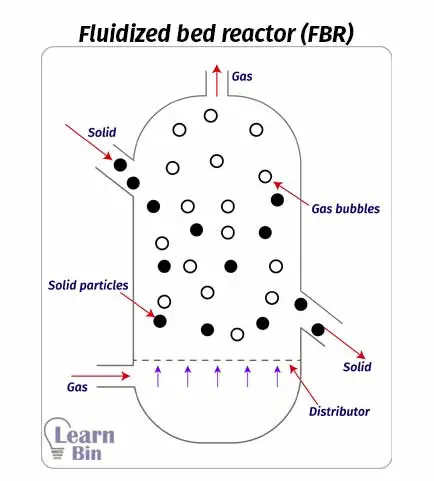
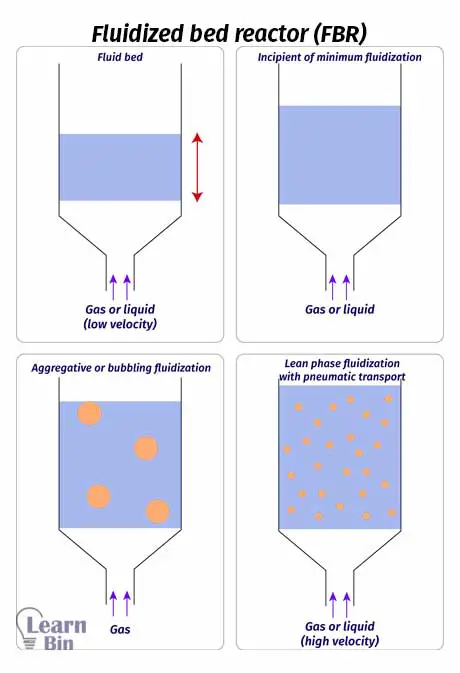
Although there are no impellers in the reactor, proper mixing is taken place due to the fluidization of solid particles. Therefore, the temperature control in FBR is better than in PBR.

The cover image was created using an image by Michael Gaida from Pixabay