More results...


The dissociation constant is a type of equilibrium constant that is used in chemical equilibrium systems of the dissociation of weak acids and bases.
When a weak acid or a weak base is dissolved in water, they don’t dissociate completely like in strong acids or bases. Only partial dissociation occurs when weak acid or weak base is dissolved in water. Because of that partial dissociation, forms an equilibrium system in the solution. The equilibrium constant associated with that equilibrium is known as the dissociation constant.
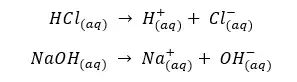
Because strong acids and bases completely dissociate in water, their equilibrium constants are larger.
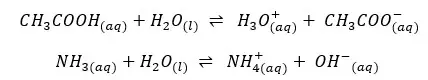
Dissociation constants (Ka and Kb) are types of equilibrium constants. So, like other equilibrium constants, Ka and Kb are temperature-dependent constants. These constants behave according to the laws of thermodynamics.
They can be used to find information such as,
But they don’t provide any information about the rate of the reaction
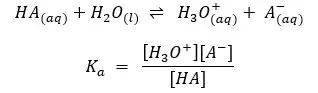
Examples:
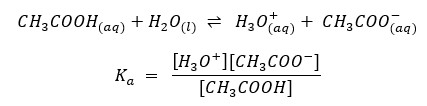
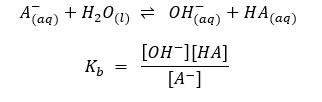
Or it can be represented like this
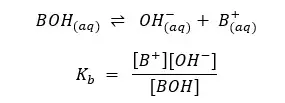
Examples:
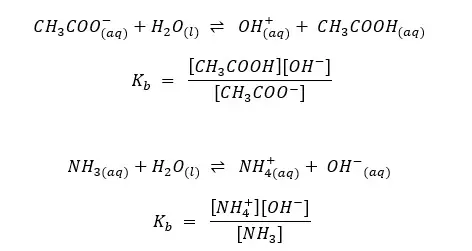
In the above examples, H2O is in every equilibrium reaction. However, it doesn’t appear in any equilibrium constant expression because [H2O] is already incorporated into the Ka and Kb.
According to the ‘Bronsted-Lowry concept’ by definition, acids donate/ release protons (H+) (proton donors) and bases accept protons (H+) (proton acceptors).
If a proton donor donates protons and becomes a proton acceptor or a proton acceptor accepts protons and becomes a proton donor, that type of species is known as a conjugated acid/base pair (conjugated pair).
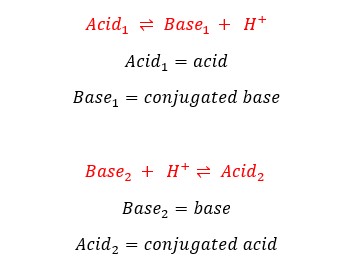
When the above two processes are combined, that forms a neutralization reaction
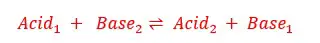
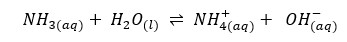
In the above system of ammonia aqueous solution, NH3 acts as a base, and water act as an acid. So NH4+ is the conjugated acid of NH3 and OH- is the conjugated base of water.
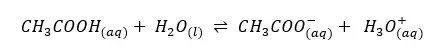
In the above system of the acetic acid aqueous solution, CH3COOH acts as an acid, and water acts as the base. So CH3COO- is the conjugated base of CH3COOH and H3O+ is the conjugated acid of water.
Let's consider an aqueous solution of ammonia (NH3) and ammonium (NH4+) ions.
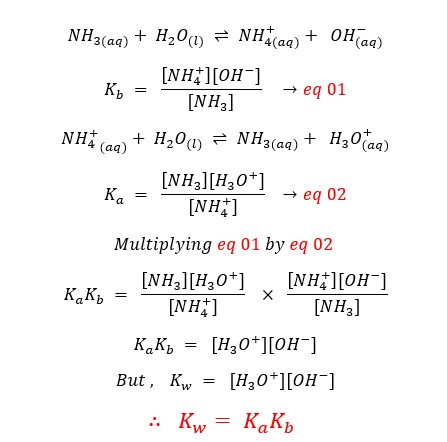
In a situation (question) you are only provided the Ka, but you need the Kb you can use the above equation to find the Kb using the above equation.
Polyprotic acids are compounds that donate more than one proton.
Polyprotic bases are compounds that accept more than one proton.
Polyprotic acids and bases have multiple Ka and Kb values that are responsible for every proton they donate or accept.
We will discuss calculations of polyprotic acids and bases (pH, Ka, Kb ...) in another article.
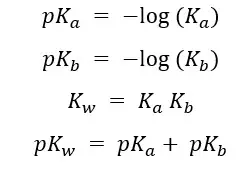
You can find out about solving problems related to pH, acid-base equilibrium system, Ka, and Kb using the article this link.

Daniel C. Harris, Quantitative Chemical Analysis; Ninth edition
Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch, Fundamentals of Analytical Chemistry; Ninth edition
The cover image was created using an image by OsloMetX from Pixabay