More results...

The molecular weight of polymers are directly affected by the various physical properties of the polymers. With the variation of the molecular weight, the physical properties such as melt viscosity, tensile strength, toughness, thermal behavior, chemical resistance, and weatherability also vary.
When a polymer has a higher molecular weight, that means it has higher physical properties. When the molecular weight is decreasing, the physical properties also decrease. Therefore, polymers with low molecular weights are undesirable for commercial applications.
Physical properties like melt viscosity, glass transition temperature (Tg), and melting temperature (Tm) are directly affected by molecular weight. Let’s consider two polymer samples of the same polymer with different molecular weights. (High molecular weight PE and low molecular weight PE)
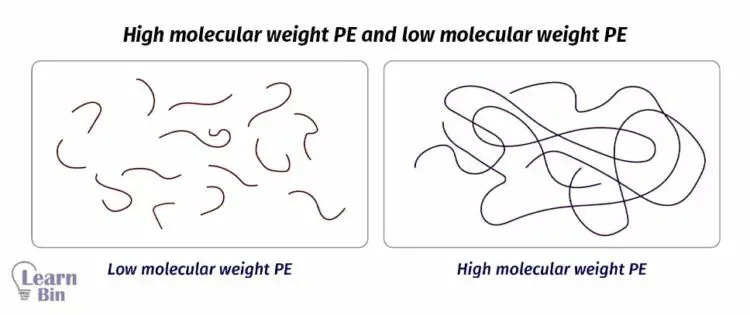
In low molecular weight PE, there are short-chain polymers. Therefore, the number of entanglements between polymer chains is low. Also, there are low intermolecular interactions between polymer chains. Because low molecular weight molecules have low surface area. So, Wander Waal’s interactions are low. In this type of system, molecules tend to behave individually.
Due to the small amount of interaction between molecules, it needs small energy to break the interactions. That means the glass transition temperature and melting temperature are low. At melt state low molecular weight polymers have less amount of entanglements. Therefore, resistance to flow under pressure is low. In other words, the melt viscosity is low.
On the other hand, in high molecular weight polymers, there are long chain molecules. So, the number of entanglements between chains and intermolecular interaction is high. It needs high energy to break these interactions. Therefore, glass transition temperature (Tg) and melting temperature (Tm) are high in high molecular weight polymers.
High molecular weight polymers show high resistance to flow in a melt state. This is because they have a higher number of entanglements between chains. Therefore, the melt viscosity is high in high molecular weight polymers. Due to the higher number of entanglements, mechanical properties such as Tensile strength (Young’s modulus), Compression resistance, and Tear resistance are also high in high molecular weight polymers.
When a certain molecular weight is reached physical properties are no longer increased. Larger molecular weight leads to higher melt viscosity. It reduces the melt flow characteristics of the polymer. Therefore, we need to optimize molecular weight for steady and continuous processing.
But molecular weight is not the only property that affects the physical properties of the polymer. The type of polymer, Branching, Tacticity, and Crystallinity also affect the physical properties.
Polymers are large molecular weight molecules. Unlike small molecules, polymers could have different molecular weights for the same polymer. The molecular weight of a polymer chain is simply the sum of the molecular weights of monomers. If there is condensation polymerization, molecular weights of small condensation molecules should be reduced.
During polymerization, polymer chains are continuously growing. Polymer chains may grow with different chain lengths. Therefore, in the same system, there are different molecules with different chain lengths (different molecular weights).
Such a system with polymer chains with a range of molecular weights is called a “Polydisperse system”. For a polydisperse system, we can obtain an average size and average molecular weight. There are four methods that represent the average molecular weight of a polymer.
Molecular weight is described as an average of the total number of polymer molecules in a unit volume. Here every polymer molecule (each chain with a different molecular weight) is treated equally. Here, we do not consider the shape or size of each polymer chain.
Number average molecular weight is calculated where certain properties depend on the number of molecules, not on the size or shape of the molecules. The properties that depend on the number of molecules are called, colligative properties such as boiling point elevation, freezing point depression, vapor pressure depression, and osmotic pressure changes.
Practically we cannot obtain a monodispersed polymer. There is a range of molecular weights in a system. To obtain the number average molecular weight, first, each molecular weight is multiplied by the number of molecules and added together. This value is divided by the total number of molecules in the system.
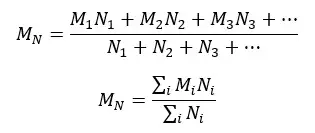
Where,
Weight average molecular weight is calculated where certain properties depend on the weight of the individual chains. Weight average molecular weight is calculated considering the larger molecules have more contribution to the average molecular weight.
In the number average molecular weight, we just consider the number of molecules. But here, we consider the size of molecules too. A property that depends on the weight average molecular weight is light scattering.
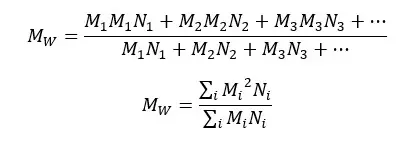
Where,
Viscosity average molecular weight is calculated for polymer solutions. Viscosity is defined as the resistance to flow. When the molecular weight of molecules is increasing, the viscosity is also increasing. This is because of the higher number of entanglements and higher friction between molecules. Viscosity average molecular weight is calculated as follows.
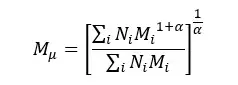
According to the Mark Howink Sakurada equation, the intrinsic of a polymer solution can be represented as follows.
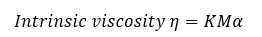
Where
These constants are determined for a given polymer solvent-temperature system. We can see how the viscosity varies with respect to the molecular weight from the following graph. This graph has been obtained by measuring viscosities for a range of molecular weights. From the following graph, we can obtain a value for α.
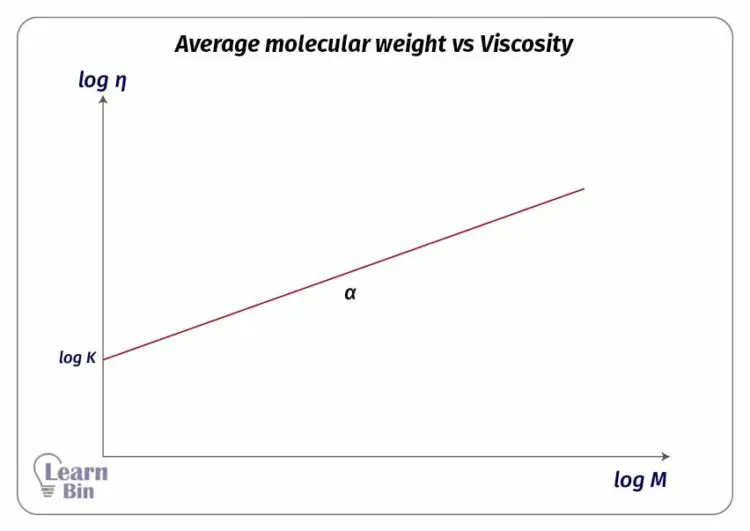
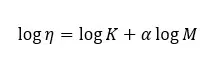
When a dilute polymer solution is applied, a low-speed centrifugal field, molecules are distributed to their molecular sizes. In this case, the molecular weights of larger polymer chains are even more important than the weight average molecular weight or viscosity average molecular weight.
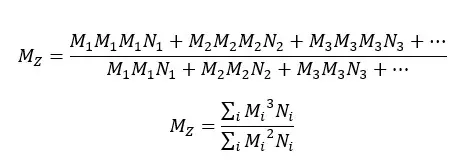
For a mono-dispersed system,
For a poly-dispersed system,
The polydispersity index is a measure of the broadness of molecular weight distribution. A larger PDI indicates broad molecular weight distribution. PDI is calculated by taking the ratio between weight average molecular weight and the number average molecular weight.
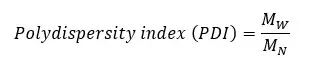
In a monodispersed system,
Therefore, for a monodispersed system, PDI is 1. The lowest value that PDI can obtain is 1.
| Polymer system | PDI |
| Monodispersed | 1 |
| Best-controlled synthetic polymer | 1.02 – 1.10 |
| Step growth polymerization | 2.0 |
| Chain growth polymerization | 1.5 – 2.0 |
The degree of polymerization is the number of repeating units in a polymer chain. It is an indicator of how long the polymer chain has grown. If the degree of polymerization is high, that means the polymer has a higher number of repeating units. In other words, the polymer chain has a high molecular weight. The degree of polymerization is calculated as follows.
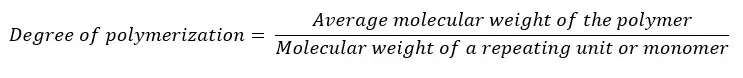

The cover image was designed using an image by Jynto, licensed under CC0, via Wikimedia Commons