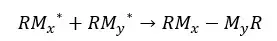More results...


There are two main types of rubber as natural rubber and synthetic rubber. We can obtain natural rubber from rubber trees. But there are some disadvantages of natural rubber. The major disadvantage of natural rubber is we cannot control its molecular weight.
In rubber trees, rubber molecules are synthesized with around 6000000 g/mol molecular weight. but the industrially important molecular weight range is 10000 – 100000 g/mol.
So, when processing natural rubber, we need additional processing steps to reduce the molecular weight. Also, the molecular weight distribution is high. Sometimes, there are some protein allergy issues with natural rubber.
To overcome the issues with natural rubber, and enhance the desired properties, synthetic rubber has been introduced. The development of synthetic rubber started back in the 19th century. Today, different commercial grades of synthetic rubber are used in the rubber industry.
According to the polymerization reaction, the synthesis of rubber can be divided into two categories.
In step-growth polymerization, all the species in the reaction medium can react with each other and form high molecular-weight species. Monomers react with each other and form oligomers. Oligomers can again react with each other to form polymers.
When reacting two oligomers to form a new molecule, the number average molecular weight of the final molecule would be the sum of the number average molecular weight of the initial oligomers.
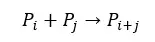
Where,
In the initial steps of the reaction, only low molecular weight products are formed. Therefore, the number average molecular weight of the product is not significantly changed. After a time, as the oligomers form polymers the number average molecular weight is rapidly increased.
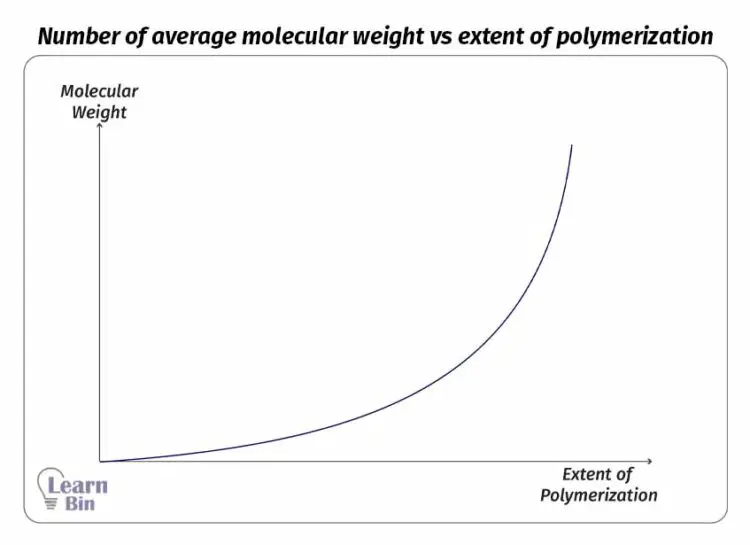
Factors that govern the molecular weight of polymers in step growth polymerization are the extent of reaction and the molar ratio of the functional groups. The maximum chain length can be controlled by adding an excess of one type of end group.
We can predict the chain length of the polymer if the initial molar ratio is known. If there is pretty much excess of one type end group, the chain length would be low. If there is an equal amount of end groups, the attainable chain length is theoretically infinite.
Step growth polymerization can be divided into two categories.
condensation reaction is a reaction where two molecules are reacted to form a single molecule and result in a small molecule as a byproduct. Usually, water results as a small molecule.
In condensation polymerization, monomer molecules are reacted a condensation manner. As an example, polyester is formed by reacting glycol and dicarboxylic acid. This reaction will result in a water molecule in each addition.
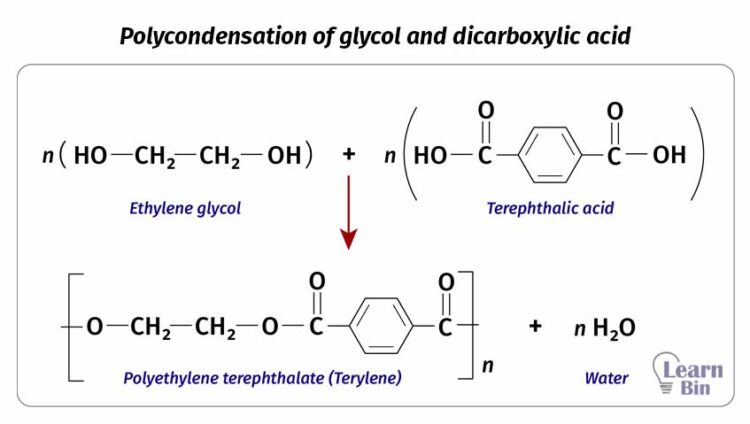
The problem with condensation polymerization is the accumulation of byproducts in the medium. Byproducts may cause depolymerization or hydrolysis of the product. Therefore, it needs to remove byproducts constantly from the reaction medium.
In polyaddition also, two molecules will react to form a new molecule. But the difference is there is no byproduct is formed in polyaddition.
e.g. - The reaction of di-isocyanates with glycols to form polyurethanes.
In chain-growth polymerization, the growth of the polymer chain occurs only by the addition of monomer to reactive sites present on the growing polymer molecules. Each macromolecule is formed by a chain reaction.
Monomers are added to the growing chain one by one.
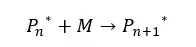
Where,
The " * " symbol indicates the reactive site of the growing chain. Monomers are added to the reactive site of the growing chain. In the initial step, the degree of polymerization is " n ". That means there are “n” number of repeating units in the chain.
After one monomer is added, the degree of polymerization will be n+1. The number average molecular weight is proportional to the extent of the reaction.
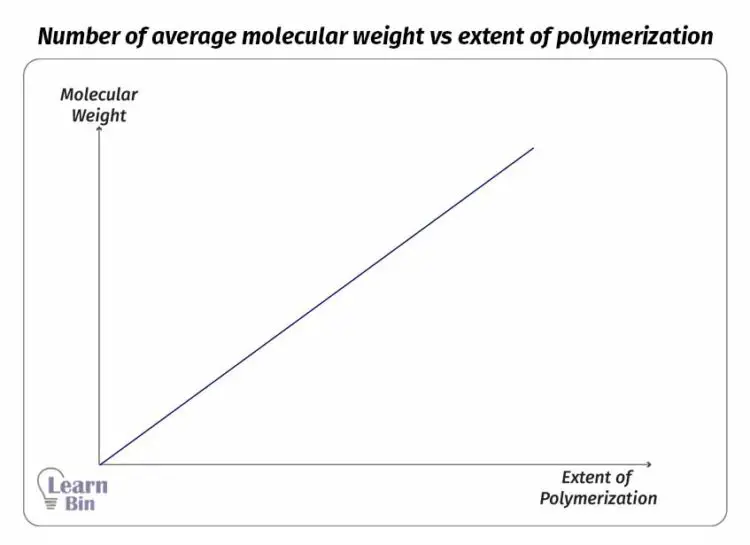
This reaction should be initiated by some reactive species. These reactive species are called initiators. Initiators have the ability to open one of the bonds in the monomer.
There are several types of initiators which are radicals, nucleophiles, electrophiles, or organometallic species. According to the initiator, there are four types of mechanisms in how the chain is formed.
There are three primary steps of free radical polymerization.
At the initiation step, free radicals are formed through the homolytic dissociation of weak bonds.
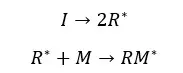
Free radicals are highly reactive. Therefore, free radicals will attack monomers and monomers will bind to the previous radical. This will result in a new radical at the end of the chain.
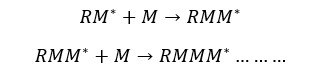
Growing chains will react to each other and form dead chains.