More results...


Polymer rheology is the study of flow behavior or the deformation behavior of a polymer. Studying the flow behavior is important because when products are manufactured from raw polymers, they should be melted. Raw polymer is in a solid state and the final product is also in a solid state. But in the intermediate state (in the process where the raw polymer is converted into products) polymer is in a liquid state.
During plastic product manufacturing, solid polymer granules are taken through a heating process and allowed to melt. Then the melted polymer is extruded or molded into a specific shape.
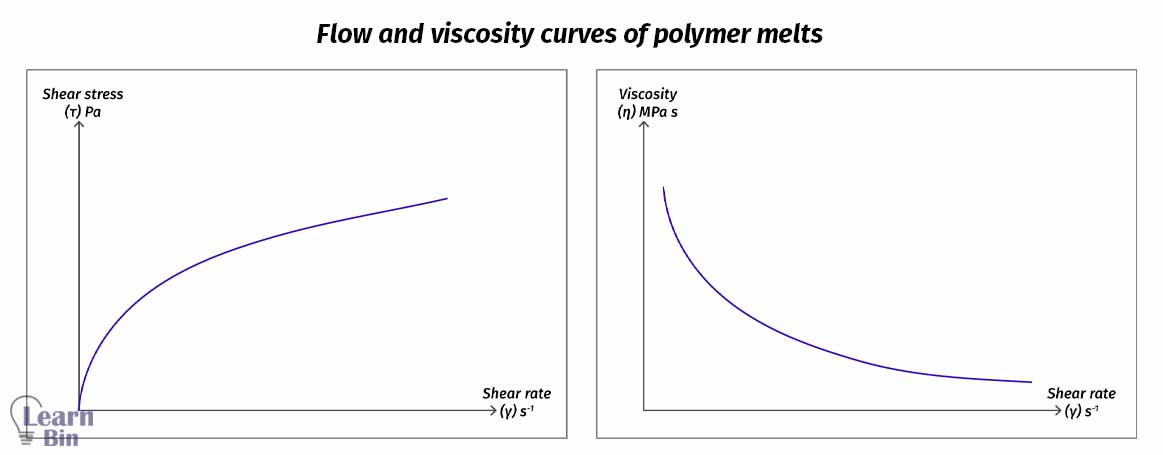
Melt flow changes its flow dynamics as we apply shear stress and change its viscosity during the process. As we improve the shear stress, viscosity varies as a function of corresponding shear strain, time, and external environmental parameters.
There is a direct correlation between chemical properties and rheological properties. Chemical properties that affect the rheology are,
Increasing molecular weight increases the weight of individual molecules and the length of the molecule. When the length of the molecule increases, the number of entanglements and the number of molecular interactions will increase. Because of these reasons, resistance to flow is also increased.
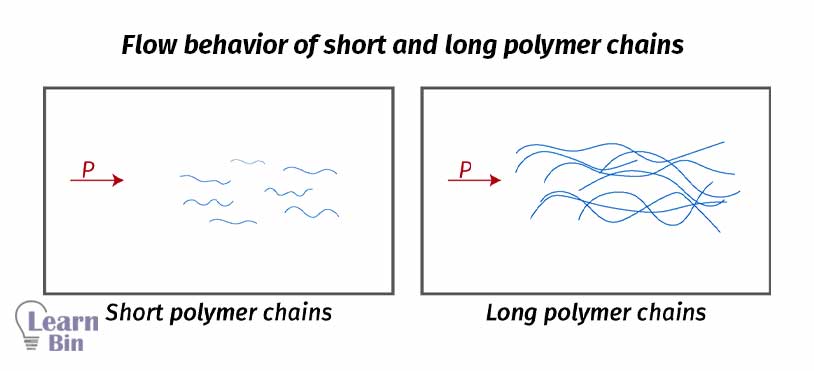
Due to the synthetic challenges, it is very hard to make polymers with a single molecular weight. Generally, polymers have molecular weight distribution. Therefore, the same polymer has different molecules with different molecular weights and different chain lengths. Hence different rheological behaviors can be obtained.
The chemical structure is the three-dimensional arrangement of a molecule in space. The same polymer can have different chemical structures.
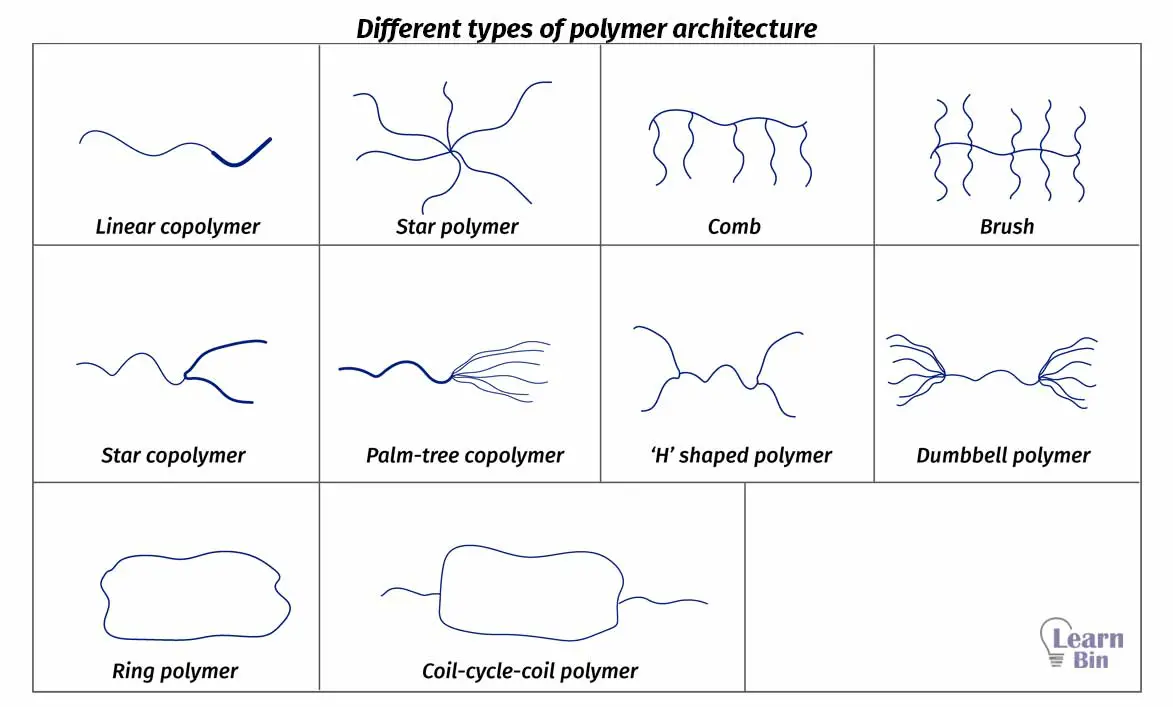
If we take two polymer samples with the same chemistry, and the same molecular weight but with different architecture, less complex architecture has a higher viscosity. As an example, linear polymers have a higher viscosity than ring polymers. Because linear polymer has a higher ability to form entanglements.
The strength of the intermolecular interactions among neighboring polymer chains determines how easily they flow at the melt stage. If the interactions are high, that means the resistance to flow at the melt stage is high.
For example, if we take polyethylene and ionomer with almost the same molecular weight, polyethylene has short-range intermolecular interactions. Because it only has Wander Waal’s interactions. Ionomers (iron-containing polymers) have strong intermolecular interactions due to dipole-dipole interactions. Therefore, ionomers are harder than PE.
Polymers are known as viscoelastic materials. Because they have viscose components as well as elastic components. Viscoelastic materials have intermediate properties between ideal solids and ideal liquids.
| Ideal solids | Ideal liquids |
| Follows Hook’s law | Follow Newton’s law |
| Stress is proportional to strain | Stress is inversely proportional to strain |
| Stores energy | Dissipates energy |
| Deformations occur | Flow |
The flow behavior of a liquid can be explained by the parallel plate model. This is the simplest model that explains the behavior of a liquid. (Especially for ideal liquids)
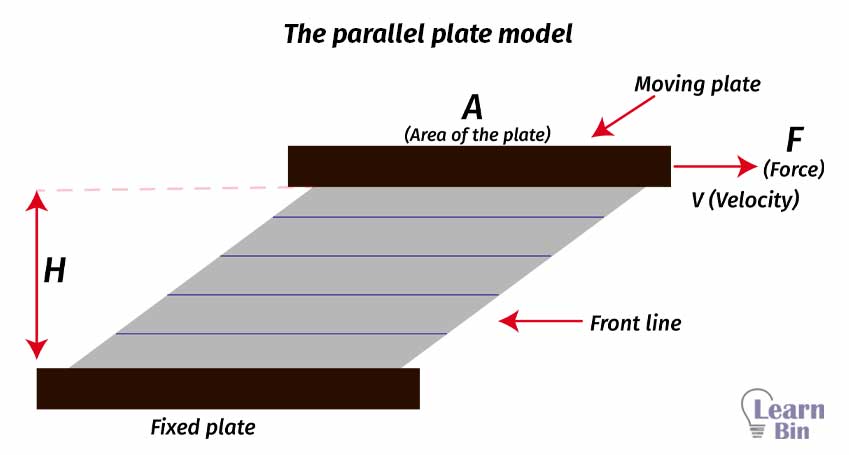
The flowing material between two moving plates is divided into layers depending on their relative speed with respect to their adjacent layer. Here, we assume that the molecular flow is laminar. That means the flow trajectories are parallel to each other and have no flow perpendicular to the main flow.
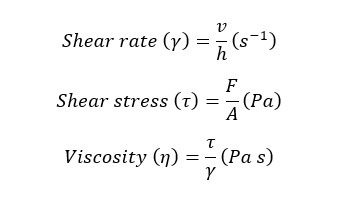
Factors affecting the viscosity

The cover image was created using an image by Rudy and Peter Skitterians from Pixabay