More results...


The most important property of elastomers/rubbers is their elasticity. Elasticity is a material property that describes the ability to stretch under the application of stress and return to its original shape when the stress is removed.
Rubbers either natural or synthetic are not 100% elastic materials. They have viscous components as well as the elastic components. Several models such as the Maxwell model, and the Einstein model have been developed to understand the elastic and viscous behavior of polymers.
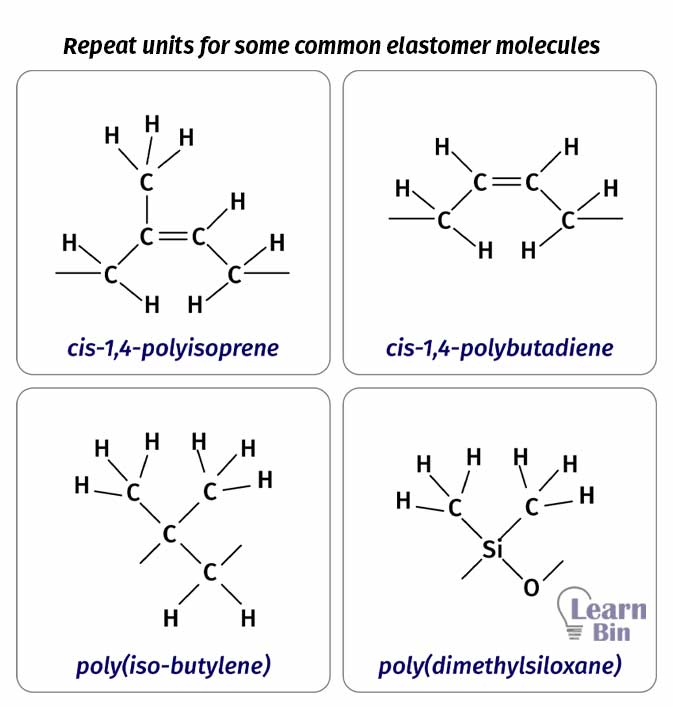
Elasticity materials consist of long flexible chainlike molecules. These rubbery molecules are made up of small molecules called monomers. Monomers are connected by valance bonds and thus a backbone is formed. The backbone of an elastomer can undergo a rapid rotation due to thermal agitation. Some repeating units of elastomers are,
When an elastomer molecule is applied a force, its end-to-end distance (r) will increase. In other words, a randomly arranged molecule is oriented under the direction of the applied force. When the force is released, the molecule comes to a random state again.
This flexibility of a single molecule comes from the double bonds in the backbone. If there are more double bonds in the backbone, the molecule would be more flexible.
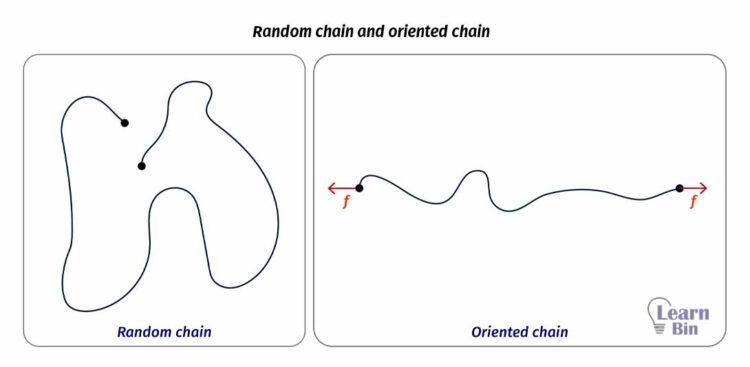
In a bulk state, the end-to-end distance of the molecules will be distributed in Gaussian statistics. Here the applied force is directly proportional to the end-to-end distance. If the average end-to-end distance is "r", and the applied force is F,
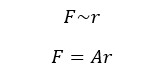
Where "A" is the proportional constant that is inversely proportional to the mean square end-to-end distance (r20)
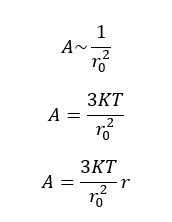
Where,
The elasticity of a 3D network of polymer molecules does not only depend on the double bonds of the backbone. In a 3D network, there are intermolecular entanglements and permanent chemical bonds called crosslinks.
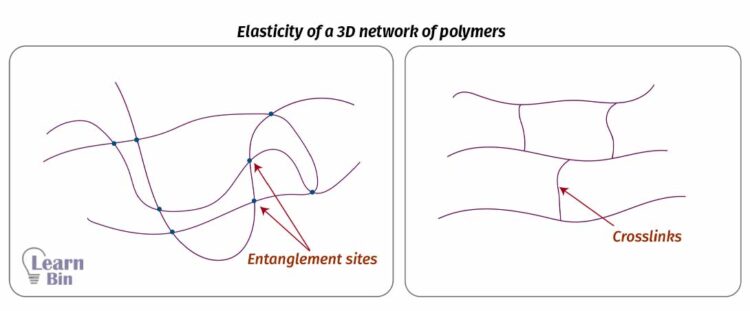
Entanglements are formed due to the molecular inter-twining in high molecular weight polymers. Therefore, some high molecular weight polymers show rubber-like behavior even though the crosslinks are absent. In a cross-linked polymer, molecules are permanently locked.
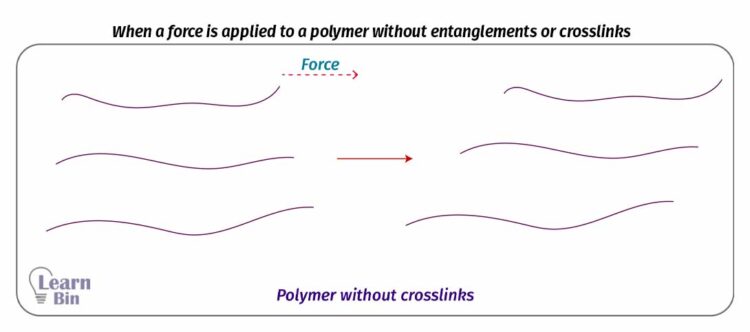
When a polymer without entanglements or crosslinks is applied a force, it will deform and when the stress is removed the polymer cannot come to its original state. But if there are crosslinks or entanglements the polymer can come to its original state when the force is removed.
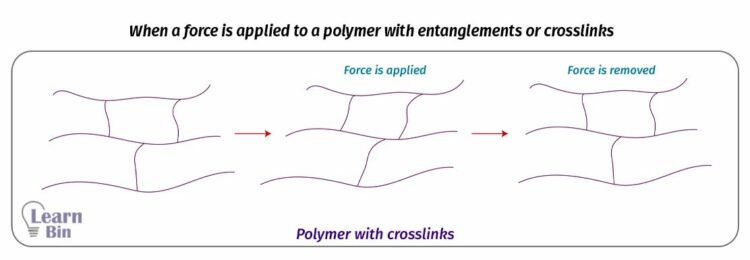
However, when the number of crosslinks is increased over a specific value, the polymer begins to become stiff. Therefore, there is an effective number of crosslinks and entanglements per unit volume.

Where,
However, the number of natural crosslinks in polymers is low. Therefore, some crosslinking agents such as Sulfur are added to the polymer network. Some representative values of the molecular weight between entanglements (Me) for some polymers are mentioned in Table 01.
| Polymer | Me (g mol-1) |
| Polyethylene | 4000 |
| cis-1,4-Polybutadiene | 7000 |
| cis-1,4-Polyisoprene | 14000 |
| Poly(isobutylene) | 17000 |
| Poly(dimethylsiloxane) | 29000 |
| Polystyrene | 35000 |

The cover image was created using an image by Donna O'Donoghue from Pixabay