More results...


Natural raw rubber or synthetic rubber cannot be directly used for product manufacturing. Before manufacturing raw materials should be compounded with specific ingredients to gain desired properties. Compounded rubber has unique characteristics such as damping characteristics, high elasticity, high strength, high stability, etc.
Rubber compounding is the process of adding additives to the rubber and obtaining a homogeneous mixture. In the compounding process, the rubber is blended with specific additives such as fillers, pigments, antioxidants, vulcanizing agents, etc., to optimize properties to meet a given set of performance requirements. There are two different grades of natural rubber raw materials dry rubber and NR latex.
The molecular weight of natural rubber can be up to 6000000 g/mol. But the commercially accepted range of molecular weight of NR is 10000 – 100000 g/mol. In this range, the physical and mechanical properties of NR are at the desired level. Therefore, the molecular weight of NR should be reduced to the optimum level before compounding.
This process is called mastication. With the reduction of the molecular weight, properties of NR such as viscosity, tensile strength, and compression strength also decreased. Mastication is done for natural rubber only. Mastication can be done either chemically (using peptizers) or mechanically. Mechanical or chemical mastication of natural rubber results, in chain session, lower molecular weight, border molecular weight distribution, and an increased number of free chain ends.
The rubber chains are mechanically broken down by using two roll mills. In two roll mill rubber is applied shear stress and heat. The heat is also caused to the thermal breakdown of the rubber chains.
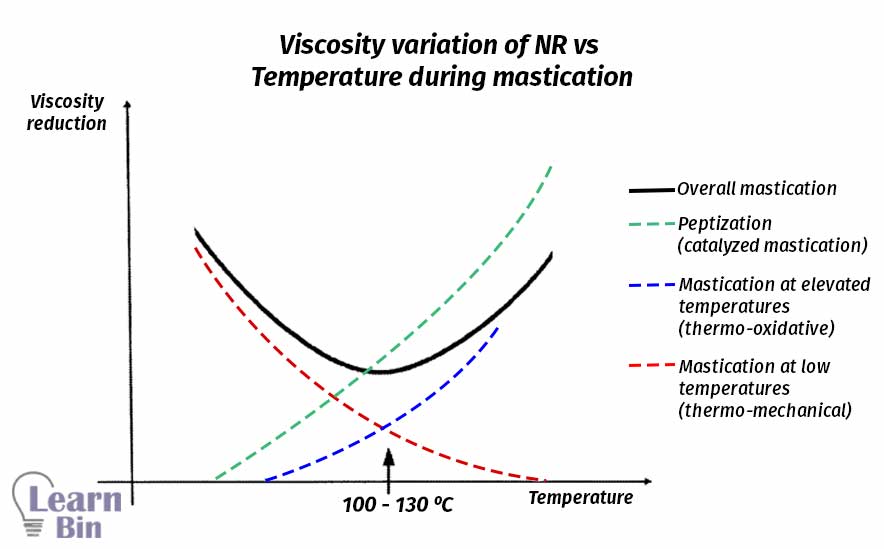
Normally, the optimum temperature for physical mastication is 60 ℃. Because at 60 ℃ rubber is still in a dry state. The temperature can be increased up to 100 ℃ to increase the efficiency of the mastication process. But with the increasing of temperature rubber will become a rubbery state and rubbery material is difficult to be processed in two roll mills.
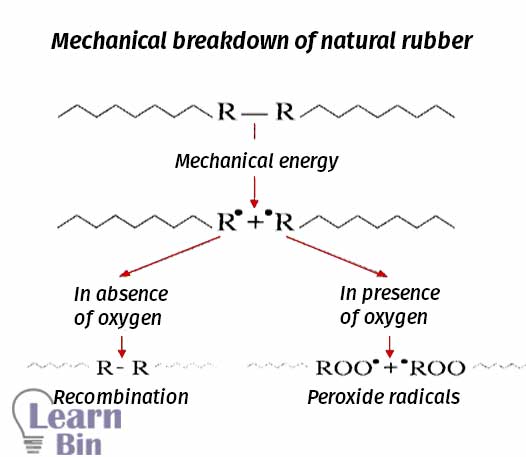
The molecular weight of NR is reduced by using chemicals. Peptizers are chemicals that are used for chemical mastication. There are two types of peptizers. Those are physical and chemical peptizers. Physical peptizers are lubricants that reduce the internal viscosity and they do not reduce the molecular weight.
Chemical peptizers are oxidation catalysts or radical acceptors. In the mastication of natural rubber, chemical peptizers prevent the recombination of polymer molecules by trapping free radicals. The benefits of the peptizing agents are
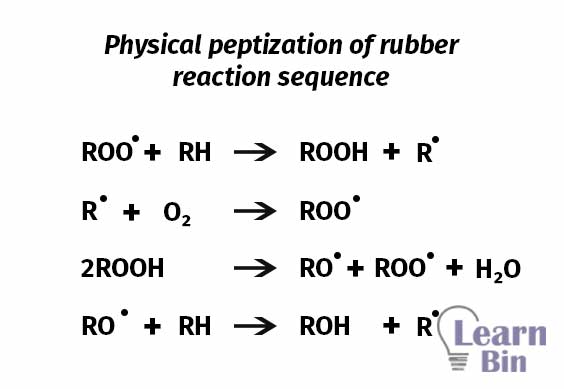
This occurs at a high rate at elevated temperatures. This process is accelerated by temperature and catalysts (peptizing agents). Peptizing agents can work as radical acceptors. Peptizing agents prevent the recombination process of the generated chain-end free radicals. The start of thermo-oxidative breakdown was shifted to much lower temperatures by all peptizing agents.
All the substances that are able to form crosslinks between the rubber chains are generally classified as vulcanizing agents. The most common vulcanizing agent uses in the natural rubber industry is sulfur. Peroxides and metal oxides are other vulcanizing agents that use in the polymer industry.
A vulcanizing system is made out of the vulcanizing agent, accelerator, and activator. Accelerators increase the speed of vulcanizing reaction while activators activate sulfur for crosslinking reaction.
The function of fillers is to fill the empty spaces of the polymer matrix. In compounding, fillers can be added up to 60%. Fillers are two types reinforcing fillers and non-reinforcing fillers. Reinforcing fillers gives reinforcement to the rubber matrix as well as cost reduction.
The most common reinforcing fillers are carbon black and silica. Carbon black acts as a reinforcing filler, antioxidant, and pigment (black color) at the same time. Non-reinforcing fillers are added to the rubber for cost reduction purposes only. Non-reinforcing fillers are also known as extenders.
E.g. - CaCO3, Clay.
Softeners are also known as plasticizers chemicals that reduce the friction between rubber molecules and increase flexibility. This will increase the plastic properties of rubber and reduce the hardness. paraffinic, aromatic, and naphthenic softeners are widely used in dry rubber technology.
Processing aids are oils that increase the fluidity or lubricant of the rubber. And these oils help to incorporate filers into polymer when milling. Processing aids and softeners increase the plastic properties of the rubber. Because during compounding rubber should have plastic nature. Therefore, processing aid is a must in compounding.
The degradation can occur in rubber either in processing or in applications due to external facts such as radiation, heat, chemicals, mechanical, etc. To prevent degradation, anti-degradants are added. The main anti degradants that use in the rubber industry are chemicals that protect the polymer from O2 and O3 attacks. Those are antioxidants and anti ozonands respectively.
Auxiliary agents are added to the rubber to incorporate desired properties in specific applications.
Blowing agents emit gases such as CO2. NH3 during vulcanization (when heating). This will result in heles in the final products. For the products like rubber slippers, and mattresses blowing agents are added in compounding. Due to the holes in the rubber matrix, it is comfortable for use.
Flame retardants are chemicals that reduce the rate of growth of fire or prevent fire.
Compounds that give color to the product.
Some rubber products such as tires, conveyor belts need extra reinforcements. For such products reinforcing textiles are used as compounding ingredients. E.g Nylon fibers.

britannica.com - Rubber chemical compound - Protective chemicals
The cover image was designed by using an image by Cjp24, licensed under CC BY-SA 3.0, via Wikimedia Commons