More results...


Polymer degradation can be categorized according to the source of energy and whether the degradation process is involved Oxygen (O2) or not.
Therefore, when the source of energy is 'heat', it is called “Thermal degradation”. When thermal degradation involves Oxygen (O2) it is called “Thermal Oxidation degradation”.
When the energy source is photo radiation, it is called “Photodegradation”. When it is involved in Oxygen (O2) it is called “Photooxidation degradation”.
High energy degradation, Mechanical degradation, and Biodegradation are other types of degradation. Biodegradation is an enzymatic reaction that happens due to the microorganisms which can initiate the biodegradation process.
The thermal degradation process is a process that is initiated by heat energy. This process does not involve Oxygen (O2). The thermal degradation process can happen in three ways. Those are depolymerization, random session, or functional group elimination.
“Depolymerization” is happening from one end of the polymer chain. In this process, monomer by monomer is removed from one end of the polymer chain. Due to the very low molecular weight of the monomer compared to the molecular weight of the polymer, the reduction of the molecular weight of the polymer is very low at the initial stage of depolymerization.
However, with time the reduction of molecular weight increases. In other words, the molecular weight decreases rapidly over time.
Polymethyl methacrylate shows a depolymerization type of thermal degradation. To reduce the depolymerization “capping agents” can be added to the end of the polymer chain. Capping agents are stable molecules that can stop the decomposition from the end. These capping agents should not have tertiary carbon.
Ex :
In a “Random session,” the polymer chain is broken down into small pieces randomly. In this process, the molecular weight decreases rapidly at the early stages of degradation. With the time reduction of molecular weight of the polymer is decreased. Polyethylene shows random session-type thermal degradation.
The other type of thermal degradation is “Functional group elimination”. In this process, functional groups in the polymer chain are removed. Polymers like PVC show functional group elimination. In the thermal degradation process of PVC, HCl molecules are eliminated. This eliminated HCl also increases the degradation process. By removing HCl, degradation can be reduced.
Polymers with high bond strength are resistant to degradation. The Resonance effect, bulky neighboring groups, and free valence in the alpha position reduce bond energy. Bonds with hetero atoms like Oxygen (O) or Nitrogen (N) with Silicon (Si), Carbon (C), or Boron (B) have high bond strength.
Polymers with crosslinks and polymers with fused rings or aromatic rings in the backbone show high thermal stability. If a polymer can form more hydrogen bonds, the thermal stability increases.
These polymers have long-term service at 300 ℃ or more. Most of these polymers have extremely high melting points and glass transition temperatures (Tg). heat resistance polymers have exceptional thermal and oxidative stability.
Nomex shows high thermal stability around 200 ℃ ~ 230 ℃. Aromatic groups on the backbone and high density of hydrogen bonds cause high thermal resistance. Nomex is used where high temperature is available, like parachutes, satellites, etc.
Polyamide structure can be stable at more than 400 ℃. Flame resistance and extraordinary mechanical properties (Tg ~ 400℃, Melting point ~ 500℃, tensile strength at 300℃~ 10000 psi). However, the disadvantage is polyamides are insoluble. Therefore, the processing is difficult.
To overcome this problem “Prepolymer solution” can be done. In the prepolymer solution process, monomers mix with each other and they do not be polymerized until the last stage of the processing stage. Monomers are easily soluble.
Polysiloxane has an inorganic backbone (Si backbone). The Silicon (Si) backbone and high density of crosslinks cause high thermal stability. A disadvantage of polysiloxane is highly brittle.
Polyacrylonitriles show the highest level of thermal stability. It is stable at around 500 ℃. Polyacrylonitrile rearranged into a ladder structure having aromatic fused rings above 500℃. Due to this structure, polyacrylonitrile achieves the highest thermal stability.
Ablative materials are organic polymers that have very low thermal conductivities and high specific heat. Most of them are composites. Generally, when these materials are heating up, a part of the polymer chars is due to the heat. That means a part of the polymer degradation happens.
Due to the degradation process, some gases are eliminated. These gases cool down the char layer and the char layer thickens. Then the rate of char decreases because the char layer acts as a protective layer.
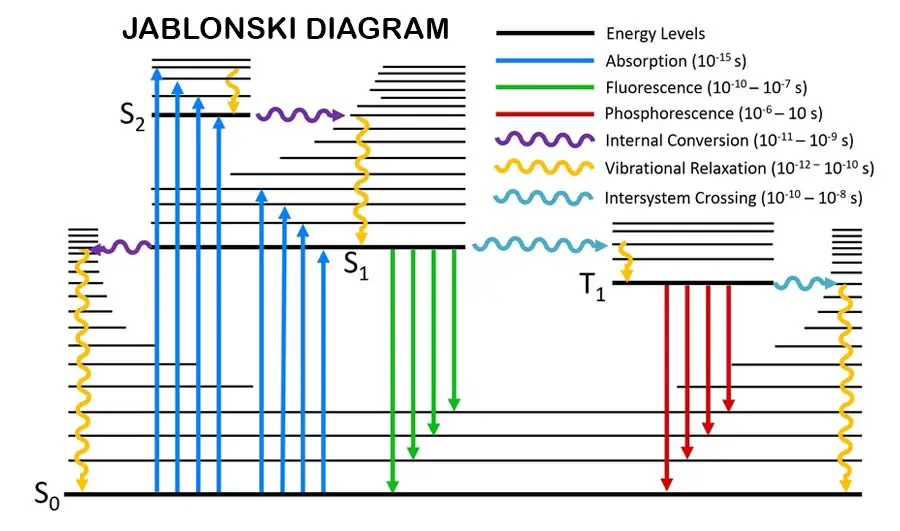
The photodegradation process is the process initiated by photo radiation. The first step of photodegradation is absorbing the photo energy. This process is called the photophysical process. Electrons in the molecules are spin-paired at the ground state. It is also called a singlet state.
There are main energy levels, vibrational energy levels, rotational energy levels, and triplet states through these main energy levels.
When the electrons absorb the energy, they can excite to higher energy levels or change their spin. When electrons change their spin, it is called “singlet to triplet transition” (electrons transition between singlet states to triplet states). There is a small energy gap between the singlet state and the triplet state.
Generally, transitions from lower energy levels to higher energy levels by absorbing energy is called “Excitation”. Excitation can happen from the ground state to main energy levels or to vibrational energy levels or rotational energy levels.
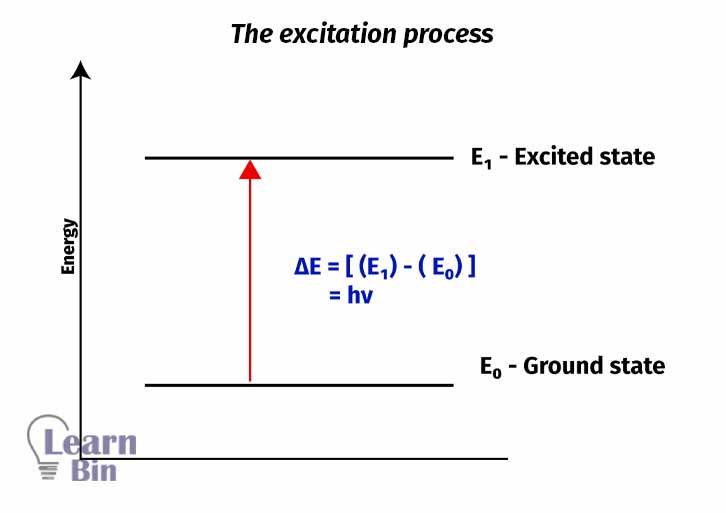
These excited electrons are very unstable due to their high energy. So, they emit their energy and come back to their original state. The emitted energy is always lower than the absorbed energy. This process can be either radiative or non-radiative.
Radiative transitions are “Florescence" and “phosphorescence”. These radiations are in the visible range in the electromagnetic spectrum. Fluorescence happens when excited electrons transition from higher main energy levels to lower main energy levels.
Phosphorescence happens when excited electrons transition from the triplet state to the lower main energy levels. Fluorescence and phosphorescence are in the visible range of electromagnetic spectroscopy.
Vibrational relaxation happens when an excited electron transitions between vibrational energy levels.
Irradiation is not shown in the photodegradation process. Absorbed energy is used for the cleavage of chemical bonds in the polymer. Due to the cleavage of bonds, free radicals are formed. These free radicals are very unstable. In other words, they are highly reactive.
So, the formed free radicals will further attack other bonds in the polymer, and ions are formed. This ionization process is called “Photoionization”. These ions undergo a cyclization reaction and form cyclic compounds. The degradation process happens continuously while happening intramolecular rearrangements and further fragmentation of the polymer. This overall process is called the “Photophysical process”.
Photooxidation is a type of degradation which is happened in the presence of oxygen (O2) or ozone (O3) and photon radiation as the energy source. The mechanism of photo-oxidation can be divided into three categories.
The primary photochemical process is a process in which the processed polymer absorbs the energy of the photon radiation by itself and decomposes the polymer by photochemical degradation. Special functional groups should be there to absorb the radiation for particular areas. Functional groups such as carbonyl groups, and unsaturated carbon bonds can absorb UV radiation.
Other ingredients such as catalytic residue, trace amount of initiators (Hydroperoxides), thermally degradative products, and other additives in the polymer can absorb UV radiation and initiate the polymer degradation.
Hydroperoxides absorb 210- 300 nm wavelengths, aldehydes absorb 270-290 nm wavelengths and ketones absorb 300 nm wavelengths in photo radiation. After absorbing the energy, these molecules undergo Norrish type 1, Norrish type 2 reactions, and cyclic reactions.
If the excited electrons do not show clear dissipation of energy, this energy can undergo a very complex series of reactions. These reactions may involve Oxygen (O2), Ozone (O3), water, acids, etc.
Learn more about polymer degradation and stability here.
Learn more about increasing the stability of polymers against degradation here.

This was a big help to my research
Thank you very much for the feedback!