More results...


Latex compounding is the process by which concentrated natural rubber latex is blended with various chemicals to get desired properties. The mixture after adding the necessary ingredient is called a compound. Natural rubber latex is preserved, concentrated, and then compounded. Synthetic rubber is directly compounded.
Stabilizers are added to the latex to increase the electrostatic stability of the latex. First, pH builders are added. pH builders are alkalis such as ammonia and KOH.
Then, surfactants are added to the latex. Surfactants that are commonly used in the latex industry are Potassium oleate, Ammonium laureate, Organic sulfates, Nonionic materials, sulphonates, etc.
Vulcanizing agents are chemicals that form crosslinks between rubber chains. The main vulcanizing agent that is used in the natural rubber industry is Sulphur. Sulfur is available in nature as eight-membered rings. These S8 rings are aggregated in Sulphur powder.
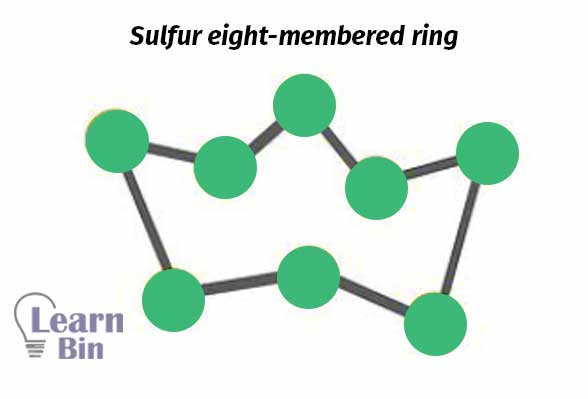

Sulfur is used for natural rubber as well as synthetic rubber containing olefinic unsaturation such as Polyisoprene styrene-butadiene rubber (SBR), nitrile butadiene rubber (NBR), butyl rubber (BR), etc. Sulfur addition to the latex is 1-2 phr (parts per hundred). For polychloroprene, Sulphur cannot be added as a vulcanizing agent. Should be added metal oxide (ZnO, Mg).
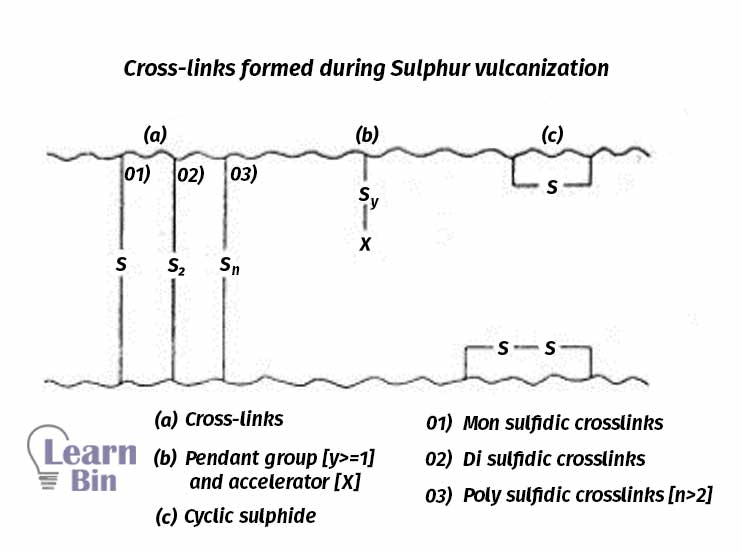
The cross-links formed during Sulphur vulcanization are of three types.
Mono and di sulfidic cross-links give better-aging resistance compared to poly sulfidic linkages. Initial properties are better for rubber vulcanizates with polysulphidic linkages. Sometimes poly sulfidic bonds break and make mono and di-sulfidic crosslinks.
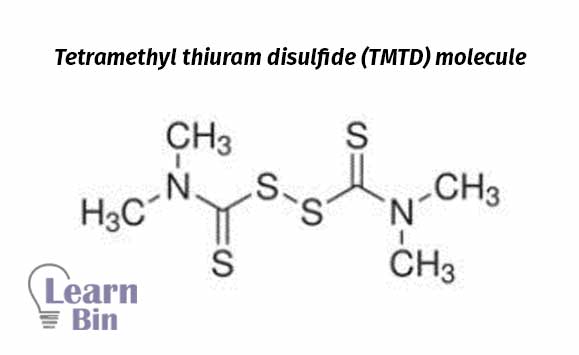
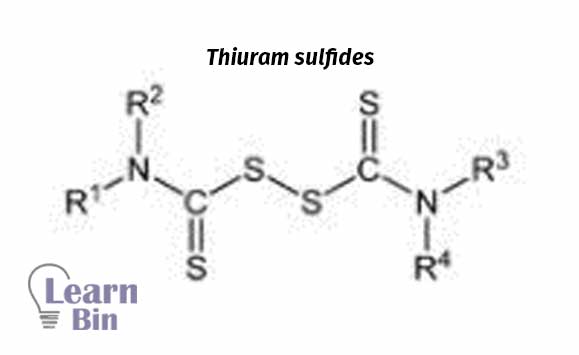
Sometimes sulfur donating chemicals (Tetramethyl thiuram disulfide (TMTD)) are used as vulcanization agents for unsaturated olefinic rubbers in the absence of sulfur or to keep low sulfur concentration to avoid sulfur staining with metals. With TMTD vulcanization temperature is relatively high (140 ℃). Sulfur vulcanization occurs at a low temp (100 ℃). At low temperatures vulcanization rate is slow.
Organic peroxides and High energy radiation are other types of vulcanizing agents.
Accelerators are compounds that increase the rate of acceleration reaction and allow vulcanization to proceed at lower temperatures with greater efficiency. Accelerators convert sulfur into a more reactive compound with rubber than sulfur does itself during vulcanization.
Vulcanizing agents are of two types - primary and secondary.
Primary accelerators directly increase the rate of vulcanization. Primary accelerators addition to the latex is 0.5 to 1.5 Phr.
Secondary accelerators are compounds that activate primary accelerators. Secondary accelerators are added to the latex only a small fraction of primary accelerators. Typically, about 0.05 to 0.5 Phr.
Dithiocarbamates are Widely used in industries as a vulcanization accelerator. These are water-insoluble and very fast accelerators.
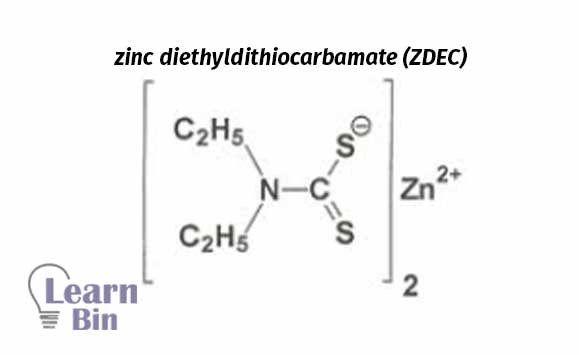
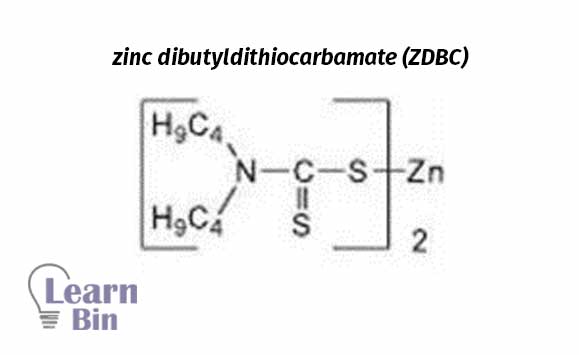
ZMBT is used as a secondary vulcanization accelerator in combination with dithiocarbamates. Very fast accelerator. These are water-insoluble and harmful to human health.
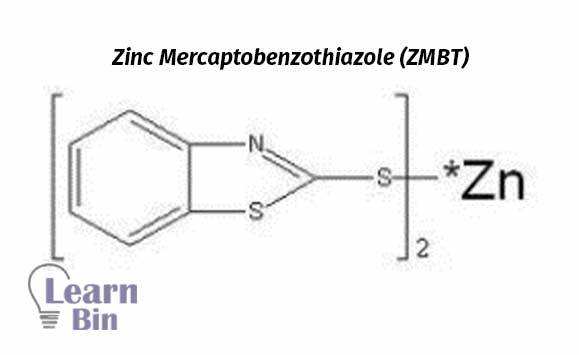
Use as a secondary vulcanization accelerator in combination with dithiocarbamates, Water-insoluble and rapidly increase the rate of vulcanization.
Vulcanizing activators are compounds that activate the acceleration reaction. The most common activator in the sulfur vulcanizing system is zinc oxide (ZnO). In a metal oxide vulcanizing system, ZnO act as a vulcanizing accelerator. Sometimes activators increase properties such as tensile strength and modulus.
Antioxidants are added to the latex to prevent the oxidative degradation of final products. There are two types of antioxidants used in the latex industry. Which are the Amine type and Phenolic type. Phenolic-type antioxidants are commonly used in the latex industry. They are water-insoluble liquids or powders. Amine-type antioxidants are not used in the latex industry, because ammine-type antioxidants give a staining effect. They are commonly used in the dry rubber industry.
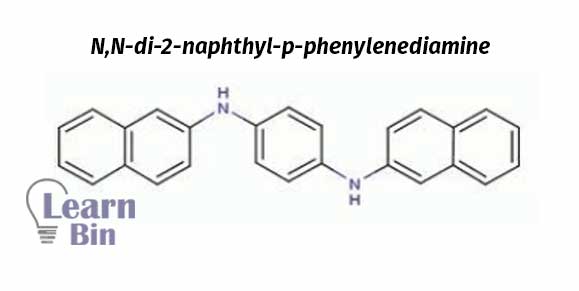
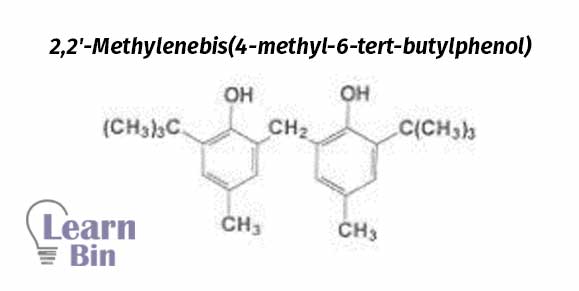
Fillers are added to latex to make it less expensive and to stiffen the product. Fillers fill the spaces in the latex matrix. Fillers increase the hardness of latex.
examples:
Pigments are compounds that give colors to latex. in the latex, industry TiO2 is added to the latex to get opaque properties from the transparent film. TiO2 gives the most effective white color to latex.
Surfactants are not compounding ingredients. These are processing ingredients. Surfactants are surface-active agents that reduce the surface tension of a liquid, the interfacial tension between two liquids, or the tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, stabilizers, and dispersing agents.
surfactants are classified as anionic, cationic, and non-ionic. Anionic surfactants are the most commonly used surfactants in the latex industry. They are cheap and perform well. Catatonics are very expensive but in certain applications, they are used as a must.
| Type of surfactant | Name | Chemical formula |
| Anionic | Sodium stearate | C17H35COONa |
| Sodium oleate | C17H33COONa | |
| Sodium laureate | C11H23COONa | |
| Sodium dodecyl sulfate | C11H25CO4SNa | |
| Cationic | Dodecyl amine hydrochloride | C12H28ClN |
| Hexadecyltrimethylammonium bromide | C19H42BrN | |
| Nonionic | Polyethylene oxides |
Viscosity modifiers or thickeners are polymeric compounds that increase the viscosity of the latex without changing its other properties. They adjust the viscosity of the latex compound over shear rates. When thin films or sheets of latex are produced, thickeners increase the thickness of the film and prevent strikethrough of the sheet.
To get desired properties the latex is compounded with different ingredients. These ingredients can be either solids or liquids. Water-soluble solids and water-miscible liquids can be easily compounded with latex and made solutions. But for the insoluble solids and immiscible liquids, dispersions and emulsions should be prepared respectively before compounding.
When preparing emulsions and dispersions, hydrophobic surfaces are converted into hydrophilic surfaces with surfactants. With the help of surfactants, water-insoluble solids can be dispersed and water-immiscible liquids can be soluble in water. The particle size of the dispersions and emulsions should be equal to the size of latex particles.
Water-insoluble solid compounding ingredients are dispersed in water and stabilized using emulsions. Sometimes the size of dispersed particles is larger than the size of latex particles. And there are some aggregated particles. Those particles should be broken down into small particles.
To reduce the size of particles different mills are used in the latex industry. There are two types of mills that are used in the preparation of dispersions. These are mills that break down aggregates of fine particles but do not reduce ultimate particle size and mills which break down aggregate and reduce the particle size of solid materials.
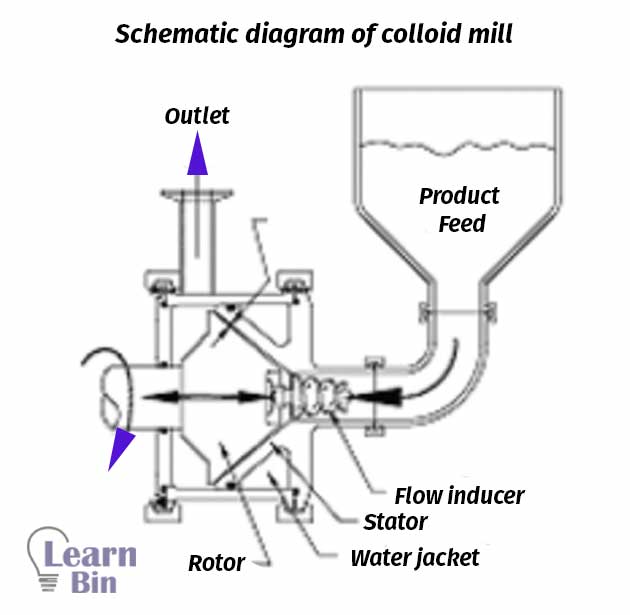
Colloid mills are used to disintegrate aggregated particles into primary particles by applying shear stress.
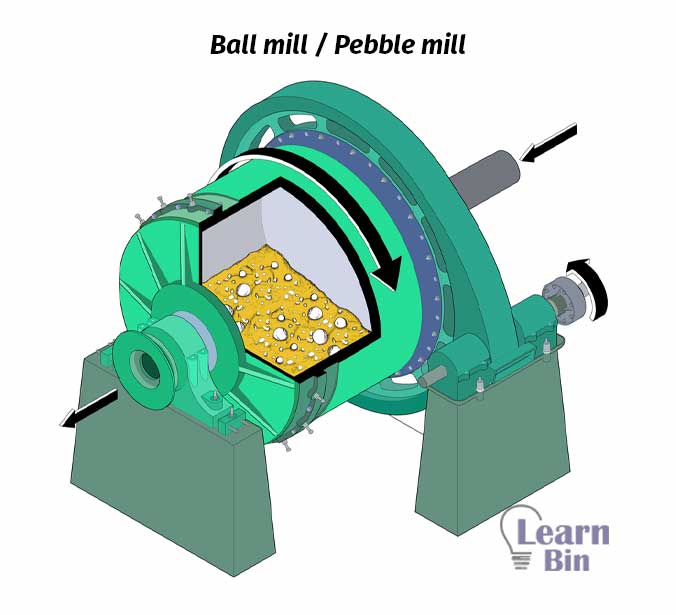
A ball mill or Pebble mill is a cylindrical type of milling machine. The hollow cylinder is filled with ceramic balls. The dispersion is filled to the cylinder and rotated. While rotating the aggregations are broken down and the size of the particles is reduced. Generally, it takes 2- 5 hours.
Attrition mills are also used to reduce the size of the solid particle in dispersion or the size of the droplets in an emulsion. The milling chamber of the attrition mill is filled with aluminum balls which supply the shear stress to the dispersion or emulsion. Generally, it takes 1-2 hours attrition mill to complete the milling process.
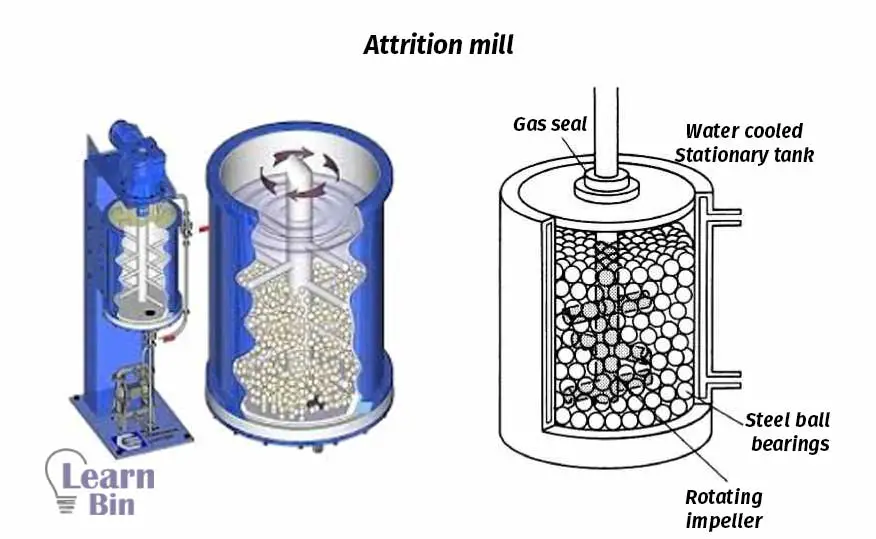
Sulfur is difficult to disperse in water because it flocculates & settles after preparation of dispersion. Therefore, a higher proportion of dispersion agents are added to the dispersion (2 - 2.5%) To stabilize sulfur, colloid stabilizers such as casein, and Bentonite clay are added. Due to the difficulty of stabilization of sulfur, it takes about 72 hours for the milling process in the Ball mill.
ZnO dispersion can be prepared either by using a ball mill or a colloid mill depending on the application. It is possible to prepare single dispersion containing all the ingredients in the ratios in which they should be present in the latex compound.
Emulsions are prepared to stabilize two or more immiscible liquids. In the latex industry emulsions are prepared to stabilize oil-type ingredients in water.
In the direct method, emulsifying agents, colloidal stabilizers (soap), and thickeners are dissolved in water. Then the oil is added to it under high-speed stirring.
The fatty acid (emulsifier) is dissolved in oil which is to be emulsified. An alkaline is dissolved in water together with any colloid stabilizer and thickeners required. Then the oil phase is added into the aqueous phase with rapid stirring
The prepared dispersions or emulsions should be stored in a container made from material that is chemically resistant to it. As far as possible the stability of dispersions & emulsion should be compatible with that of latex. Furthermore, it is better if the stabilizing systems are similar. As far as possible the pH of the solutions, dispersions & emulsions should be adjusted to that of the latex to which they are added.

polymerdatabase.com - Accelerators for Sulfur Vulcanization of Rubbers
Polymer Latices: Science and Technology Volume 3: Applications of latices - Blackley.D. - 1997 - Dordrecht: Springer Netherlands.
pharmapproach.com - Colloid mill
The cover image was designed using a photo by x3, available in pixabay
Figure 13: Image by Rusch, Heather, creator, licensed under Public domain, via Wikimedia Commons
Nice and very clear explanation,
Actually this helped me a lot to write my industrial training report.